Corneal ectasia can severely impair vision, especially in the progressive form caused by the inherent structural instability of the cornea. The clinical signs can challenge your detection skills—corneas with subclinical keratoconus are clinically normal in appearance but have subtle irregularities on corneal topography. These cases are especially important to identify because they can easily develop into iatrogenic keratoconus post refractive surgery.1
Advanced corneal imaging systems are detecting earlier stages of genetically caused ectasias and also minimizing iatrogenic causes of ectasias from LASIK and other corneal surgery procedures.2
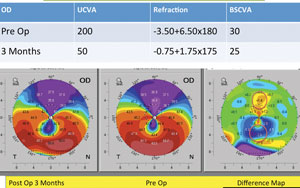
 | |
| Pentacam imaging of a 68-year-old male patient with keratoconus. Image: William Trattler, MD. |
Ectasias can also present from contact lens-induced corneal warpage and, while distortion is sometimes permanent, usually these are temporary and return to normal once the CLs are removed. Vision correction for ectasias has been improving through advanced gas permeable/scleral CLs and medically through collagen crosslinking and lamellar surgeries. Regardless of the cause, treatments to improve the vision of ectasias are similar.
Although treatments are numerous, those within optometry’s purview are mostly limited to corrective lenses; still, we are called upon to diagnose and comanage these patients throughout the course of the disease, which can span decades.
For these reasons and more, all optometrists should be well versed in the causes and consequences of corneal ectasia. This article will review current technology for detection, CL corrections for visual symptoms and the range of medical and surgical treatments available.
Causes of Ectasias
Ectasias are either genetically or iatrogenically caused. The most prevalent and most understood genetically caused ectasia is keratoconus. Affecting approximately 50 to 230 per 100,000, keratoconus is a non-inflammatory corneal disease with clinical signs of inferior corneal progressive stromal thinning, scissor reflex upon retinoscopy, corneal protrusion and irregular astigmatism.3-5 Histopathologically, there is iron deposition in the epithelial basal layer and breaks in Bowman’s layers.6 Though typically they present bilaterally asymmetrical, reports of unilateral keratoconus cases exist.7 The second eye of unilateral keratoconus is still considered a suspect for developing keratoconus and is hence contraindicated for LASIK.
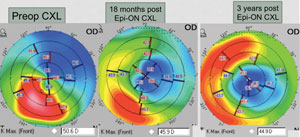
 | |
| Pentacam imaging of a 38-year-old male with post-LASIK ectasia. The three images detail changes post epi-on crosslinking treatment. Image: William Trattler, MD. |
Iatrogenic ectasias occur primarily through post-corneal refractive surgery. They are rare, but debilitating when they occur. Studies have reported incidence rates of 0.04% to 0.66%, with higher complications post LASIK as compared to photorefractive keratectomy (PRK).1,8-10 Iatrogenic ectasias are generally found in refractive surgery patients that are younger, have high myopia, have thinner corneas or have had multiple refractive surgery enhancements.1,11 Thicker flaps, which compromise residual stromal thickness, excessive tissue removal from high refractive errors and/or undiagnosed forme-fruste keratoconus during LASIK, or both, ultimately causes thinner corneas. The thinned corneal biomechanical strength wanes while the intraocular pressure causes forward corneal bowing, resulting in ectasia.12
One study found that all patients who develop iatrogenic ectasia post LASIK had at least one predictable risk factor.10 Studies have reported post-LASIK ectasia development from three to 57 months after the surgical procedure.10,13
Detection of Ectasia
Whereas moderate and severe ectasias are easily diagnosed with biomicroscopy, retinoscopy and traditional placido disc-based corneal topographies linked to programmed indices and algorithms specifically designed for keratoconus detection and diagnosis, subclinical keratoconus is more difficult to detect.14-18 The popularity of LASIK and other forms of corneal refractive surgery in the 1990s and the risk of iatrogenic ectasia spurred the development of corneal tomography, which has revolutionized the detection of subclinical keratoconus.
| Table 1. Treatment Options for Keratectasia | ||||
| Ectasia Severity | Early | Moderate | Advanced | |
| CL Treatments | Glasses Soft CLs | Corneal GPs Piggyback CLs Hybrid CLs Scleral lenses | Corneal GPs | |
| Surgical Treatments | CXL Intracorneal rings | CXL Intracorneal rings | Lamellar keratoplasty PKP | |
Corneal tomography produces three-dimensional images of the cornea from two-dimensional cross sections. The modality includes slit scanning, Scheimpflug imaging, OCT and very high frequency (VHF) ultrasound imaging. Each of these devices, when used independently, gathers different information about the cornea. They each evaluate the anterior and posterior corneal elevations and their pachymetric distribution, which is important for detecting any ectasia or predisposition to ectasia. When the information from different devices is used together, the accuracy of ectasia detection is even greater.
• Slit scanning involves slits projected on the cornea. The anterior and posterior edges of the slits are analyzed and presented as three-dimensional topographic maps. Research shows this is more sensitive than earlier devices for detection of keratoconus since it takes images of the entire cornea.19-21 The latest slit scanning systems, including the Orbscan II, actually use both slit scanning and placido technology.
• The rotating Scheimpflug imaging devices such as the Pentacam (Oculus) measures the anterior and posterior corneal surfaces, as well as corneal volume and spatial profiles from three-dimensional models. Researchers found that keratoconus has thinner corneas with less corneal volume.22
• Optical coherence tomography creates an optical cross sectional scan of the specific layers of the cornea. This could be important for detecting epithelial thickness irregularities, which may be early indicators of early keratoconus.23
• VHF ultrasound measures the thickness of individual corneal layers by a series of scans in different meridians in an arc that matches the curvature of the cornea. Researchers used this to measure the mean thickness of LASIK flaps and found that those with a mean thickness of 163.6µm had greater risks of ectasia, especially in patients with greater ablation depth.24
Treatment of Ectasias
The first-line treatment for visual disturbance due to corneal ectasia is corrective lenses, but recent surgical advances offer the promise of more permanent solutions.
• Contact lenses. In early stages of ectasia, spectacles, soft CLs and even custom aberration-correcting soft CLs may be adequate to correct for vision changes. But as the ectasia progresses, the optically smooth surface from a rigid gas permeable (GP) lens is necessary to ameliorate the irregular corneal surface of the ectatic eye to provide clearer vision. Corneal GPs, and now more popularized scleral lenses, are the mainstay visual treatments for these eyes.25 GPs comprise 65% of contact lens correction for keratoconus and has delayed the need for surgery in approximately 80% to 98.9% of all fittings.3,26,27
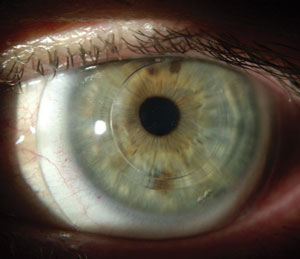 | |
| Intacs in an eye with keratoconus. Photo: William Trattler, MD. |
Corneal GPs range in size from 8mm to 10mm in diameter and are ideal for small central cones or mild keratoconic eyes.28 With increased ectasia severity, larger diameters improve lens centration. Intralimbal lenses are slightly larger at 10.5mm to 12mm in diameter. Though corneal GPs provide crisper vision than soft lenses, dropout occurs from discomfort. Piggyback CLs can improve comfort, but can be inconvenient for patients because of their need to clean and care for both soft and GP contact lenses. Hybrid CLs may also improve the comfort; however, their variable clinical performance, high giant papillary conjunctivitis rates and breakage at the GP and soft lens junction may limit their use.29,30 The very recent surge of scleral lens options and popularity has provided a more comfortable option for ectatic eyes.
Scleral lenses are generally reserved for moderate to advanced stages of ectasia as well as those who have failed in comfort from traditional GPs. Whereas corneosclerals (12.9mm to 14.9mm) rest on both the cornea and sclera, minisclerals (15.0mm to 18.0mm) and full scleral lenses (18.1mm to 24.0mm) rest entirely on the conjunctiva and vault over the cornea.28,31 Larger lenses are more comfortable especially for those with focally steep cones or those with sensitive corneal epithelium since vaulted lenses reduce friction with the cornea.28,32 Scleral lenses should be fit with the highest Dk material possible and without excessive corneal clearance, to minimize hypoxia from the combination lens and tear film thickness.33
• Medical/surgical treatment. For ectatic patients who are intolerant of CLs, medical and surgical treatments are available. Traditional treatments have been limited to contact lenses and full-thickness penetrating keratoplasty (PKP). Though graft survival rates usually extend up to 20 years and sometimes beyond, reasons to defer doing PKPs for as long as possible include the generally young age of keratoconic patients (and thus the challenge of achieving life-long graft survival), graft rejection and failure, surgical complications and the risks of developing secondary cataracts and glaucoma from long-term steroid use. Visual rehabilitation after PKP is also difficult.
• Intrastromal corneal ring segment implantation. For mild to moderate ectasias with little to no corneal scarring, intrastromal corneal ring (Intacs) use is an option. It involves the insertion of one or two polymethylmethacrylate segments into the corneal stroma to flatten the irregular anterior corneal shape and hence improve uncorrected visual acuity.34 Segments of varying thickness can be implanted; the thicker the segment, the more significant the flattening effect. They are generally inserted on the inferior cornea to flatten the steep areas of inferiorly located cones. Intrastromal corneal rings do not stop the progression of keratoconus and the residual refractive errors are often more challenging to treat with corneal GP contact lenses due to the sharp topography differences at the junction of the intrastromal corneal ring segment.35 Complications many include patient dissatisfaction with visual outcomes, discomfort and ring segment extrusion.36
• Collagen crosslinking (CXL). Whereas the current treatments for ectasias revolve around visual rehabilitation, CXL is a promising treatment to actually delay and potentially halt the progression of many ectasias, including keratoconus, pellucid marginal degeneration and post-LASIK ectasias.37-40 It is a procedure that increases corneal rigidity and biomechanical stability by forming new chemical bonds between the collagen strands of the corneal stroma. This is a procedure that would most benefit early to moderate ectasias.
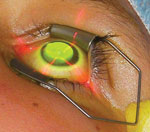 | FDA Approval Pending for US Launch of Crosslinking for Keratoconus Though its advisory panel recommended approval, the FDA recently requested additional information before giving the go-ahead to Avedro’s drug/device combination of Photrexa (riboflavin) and KXL System (UVA light) for the treatment of progressive keratoconus and corneal ectasia following refractive surgery. The company is working to address the questions and move forward with FDA approval. | |
| A keratoconic patient undergoing a collagen crosslinking procedure. Photo: Peter Hersh, MD, Hersh Vision Group, Teaneck, NJ. |
CXL involves the application of riboflavin on epithelialized or de-epithelialized central corneas, depending upon protocol treatment. The cornea is then exposed to ultraviolet A light, which activates the riboflavin and creates new crosslinks within the collagen and intrastromal matrix of the stroma.40 CXL on de-epithelialized corneas is more effective than on epithelized corneas, but there is a longer recovery time, more patient discomfort and increased risk of infections.37,41
Accelerated CXL uses a higher intensity UV light for shorter periods of time. Studies are evaluating the effectiveness and safety profile of accelerated vs. non-accelerated CXL. Evidence may show that the accelerated CXL is as effective and safe as conventional CXL, but more research is needed.42 Though rare, risks of complications post CXL include corneal haze, keratitis, endothelial cell loss and CXL ineffectiveness.39,40
Long-term studies are limited, but show promise in achieving ectasia stability in many patients. Researchers found the standard CXL method improved visual acuities and stabilized the progression of post-LASIK induced ectasias over 42 months.40 In a retrospective case series, another study found that accelerated CXL was able to halt the progression of keratoconus, in addition to improving their patients’ visual acuities, keratometry values and corneal aberrations after 24 months.39 Overall, CXL is a novel treatment to stabilize the progression of both keratoconus and post-LASIK ectasia.
Clinical Pearls
|
Combining other interventions with CXL offers a new frontier in treatment that addresses both structural and refractive needs. While intrastromal corneal rings improve the visual acuity, CXL performed after intrastromal corneal ring implantation stabilizes the cornea to slow down future progression.43 One study showed the addition of the corneal crosslinking after intrastromal corneal ring insertion improved the keratoconic outcomes more than corneal crosslinking alone.44 But caution is warranted since keratoconus can still progress years after corneal crosslinking.45,46
• Lamellar keratoplasty. When the above treatments fail, severe ectasias may require surgical intervention. Lamellar procedures such as deep anterior lamellar keratoplasty (DALK) and crescentic lamellar keratoplasty are options. DALK involves removing the corneal stroma down to Descemet’s membrane and replacing it with donor cornea with or without Descemet’s membrane. Compared to PKP, DALK offers a lower risk of graft rejection, early visual rehabilitation, better wound strength and limited endothelial cell loss. It is a complicated procedure, but one with the same rate of graft survival and final visual acuity outcomes as PKP.47
For ectasias that involve the far periphery of the cornea, such as in pellucid marginal degeneration, large-diameter PKPs are discouraged as their proximity to the limbus and its blood vessels make grafts more prone to rejections.
Semilunar, crescentic and annular lamellar keratoplasty use donor grafts that spare the central cornea. The central vision is minimally affected even if the graft is rejected and becomes opaque. These grafts decrease the overall corneal astigmatism, but the results can be short-lived because thinning and ectasia can recur.48,49
Advancements in ectasia detection, prevention and treatment are constantly emerging. Improved technologies, especially in the form of corneal tomography, can now identify genetically caused ectasias at early stages. The combination of the different tomography systems provides complementary views of the cornea to hopefully provide a better understanding of the predictors of iatrogenic ectasia and ultimately eliminate that as well. Ectasia patients are fortunate to have growing visual rehabilitation treatment options in the advancing areas of contact lenses, collagen crosslinking, intrastromal corneal ring implantation and lamellar surgeries. Future combination treatments with CXL will hopefully one day eliminate the need for corneal transplantation.
Dr. Yeung is a diplomate in the Cornea, Contact Lenses, and Refractive Technologies section of the American Academy of Optometry. She is the senior optometrist at UCLA Arthur Ashe Student Health and Wellness and a clinical assistant professor of Western University of Health Sciences College of Optometry.
Sally Wu is a pre-optometry student getting a BA in psychology at UCLA, class of 2015.
1. Randleman JB, Woodward M, Lynn M, et al. Risk assessment for ectasia after corneal refractive surgery. Ophthalmol. 2008;115:37-50.2. McMonnies CW. Screening for keratoconus suspects among candidates for refractive surgery. Clin & Exp Optom. 2014;97:492-8.
3. Kennedy RH, Bourne WM, Dyer JA. A 48-year clinical and epidemiologic study of keratoconus. Am J Ophthalmol. 1986;101:267-73.
4. Rabinowitz YS. Keratoconus: major review. Survey of Ophthalmol. 1998;42(4):297-319.
5. Romero-Jimenez M, Santodomingo-Rubido J, Woffsohn JS. Keratoconus: a review. Cont Lens Anterior Eye. 2010;33:157-66.
6. Sherwin T, Brookes, NH. Morphological changes in keratoconus: pathology or pathogenesis. Clin Experiment Ophthalmol. 2004;32(2):211-7.
7. Li X, Rabinowitz YS, Rasheed K, et al. Longitudinal study of the normal eyes in unilateral keratoconus patients. Ophthalmology. 2004;111(3):440-6.
8. Rad AS, Jabbarvand M, Saifi N. Progressive keratectasia after laser in situ keratomileusis. J Refract Surg. 2004;20(5):S718-22.
9. Pallikaris IG, Kymionis GD, Astyrakakis NI. Corneal ectasia induced by laser in situ keratomileusis. J Cataract Refract Surg. 2001;27(11):1796-802.
10. Randleman JB, Russell B, Ward MA, et al. Risk factors and prognosis for corneal ectasia after LASIK. Ophthalmology. 2003;110:267-75.
11. Holland SP, Srivannaboon S, Reinstein DZ. Avoiding serious corneal complications of laser assisted in situ keratomileusis and photorefractive keratectomy. Ophthalmol. 2000;107:640-52.
12. Ambrosio R Jr, Nogueira LP, Caldas DL, et al. Evaluation of corneal shape and biomechanics before LASIK. Int Ophthalmol Clin. 2011;51:11-39.
13. Said A, Hamade JH, Tabbara KF. Late onset corneal ectasia after LASIK surgery. Saudi J Ophthalmol. 2011;25:225-30.
14. Kalin NS, Maeda N, Klyce SD, et al. Automated topographic screening for keratoconus in refractive surgery candidates. CLAO J. 1996;22:164-7.
15. Maeda N, Klyce SD, Smolek MK, et al. Automated keratoconus screening with corneal topography analysis. Invest Ophthalmol Vis Sci. 1994;35:2749-57.
16. Maeda N, Klyce Sd, Smolek MK. Comparison of methods for detecting keratoconus using videokeratography. Arch Ophthalmol. 1995;113(7):870-4.
17. Rabinowitz YS, McDonnell PJ. Computer-assisted corneal topography in keratoconus. Refract Corneal Surg. 1989;5:400-8.
18. Rabinowitz YS, Rasheed K. KISA% index: a quantitative videokeratography algorithm embodying minimal topographic criteria for diagnosing keratoconus. J Cataract Refract Surg. 1999;25(10):1327-35.
19. Lim L, Wei RH, Chan WK, et al. Evaluation of keratoconus in Asians: role of Orbscan II and Tomey TMS-2 corneal topography. Am J Ophthalmol. 2007;143(3):390-400.
20. Reddy JC, Rapuano CJ, Cater JR, et al. Comparative evaluation of dual Scheimpflug imaging parameters in keratoconus, early keratoconus, and normal eyes. J Cataract Refract Surg. 2014;40:582-92.
21. Sahin A, Yildirim N, Baskmak H. Two-year interval changes in Orbscan II topography in eyes with keratoconus. J Cataract Refract Surg. 2008;34(8):1295-9.
22. Ambrosio R, Alonso RS, Luz A, et al. Corneal-thickness spatial profile and corneal-volume distribution: tomographic indices to detect keratoconus. J Cataract Ref Surg. 2006;32:1851-9.
23. Kanellopoulos AJ, Asimellis G. OCT corneal epithelial topographic asymmetry as a sensitive diagnostic tool for early and advancing keratoconus. Clin Ophthalmol. 2014;18(8):2277-87.
24. Reinstein DZ, Sutton HF, Srivannanboon S, et al. Evaluating microkeratome efficacy by 3D corneal lamellar flap thickness accuracy and reproducibility using Artemis VHF digital ultrasound Arc-Scanning. J Refract Surg. 2006;22:431-40.
25. Weed KH, MacEwen CJ, McGhee CN. The Dundee University Scottish Keratoconus Study II: a prospective study of optical and surgical correction. Ophthalmic Physiol Opt. 2007;27(6):561-7.
26. Wagner H, Barr JT, Zadnik K. Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study: methods and findings to date. Cont Lens Anterior Eye. 2007;30(4):223-32.
27. Bilgin LK, Yilmaz S, Araz B, et al. 30 years of contact lens prescribing for keratoconic patients in Turkey. Contact Lens Ant Eye. 2009;32:16-21.
28. Barnett M, Mannis J. Contact lens in the management of keratoconus. Cornea. 2011;30(12):1510-6.
29. Maguen E, Caroline P, Rosner IR, et al. The use of the SoftPerm lens for the correction of irregular astigmatism. CLAO J. 1992;18(3):173-6.
30. Abdalla YF, Elsahn AF, Hammersmith KM, et al. SynergEyes lenses for keratoconus. Cornea. 2010;29(1):5-8.
31. Worp EVD, Bornman D, Ferreira DL, et al. Modern scleral contact lenses: A review. Contact Lens & Anterior Eye. 2014;37:240-50.
32. Schornack MM, Patel SV. Scleral lenses in the management of keratoconus. Eye & Contact Lens. 2010;36(1):39-44.
33. Jaynes JM, Edrington TB, Weissman BA. Predicting scleral GP lens entrapped tear layer oxygen tensions. CLAE. 2015;44-7.
34. Zare MA, Hashemi H, Salri MR. Intracorneal ring segment implantation for the management of keratoconus: safety and efficacy. J Cataract Refract Surg. 2007;33:1886-91.
35. Hladun L, Harris M. Contact lens fitting over intrastromal corneal rings in a keratoconic patient. Optometry. 2004;75(1):48-54.
36. Pinero DP, Alio JL, Uceda-Montanes A, et al. Intracorneal ring segment implantation in corneas with post-laser in situ keratomileusis keratectasia. Ophthalmol. 2009;116(9):1665-74.
37. Lesniak SP, Hersh PS. Transepithelial corneal collagen crosslinking for keratoconus: Six-month results. J Cataract Refact Surg. 2014;40:1971-9.
38. Moshirfar M, Edmonds JN, Behunin NL, et al. Current options in the management of pellucid marginal degeneration. Journal of Refractive Surgery. 2014;30(7):474-85. 39. Ozgurhan EB, Kara N, Cankaya KI, et al. Accelerated corneal cross-linking in pediatric patients with keratoconus: 24-Month Outcomes. Journal of Refractive Surgery. 2014;30(12):843-9.
40. Yildirim A, Cakir H, Kara, et al. Corneal collagen crosslinking for ectasia after laser in situ keratomileusis: Long-term results. J Cataract Refract Surg. 2014;40:1591-6.
41. Hashemiman H, Jabbarvand M, Khodaparast M, et al. Evaluation of corneal changes after conventional versus accelerated corneal cross-linking: A randomized controlled trial. Journal of Refractive Surgery. 2014;30(12):837-42.
42. Konstantopoulos A, Mehta JS. Conventional versus accelerated collagen cross-linking for keratoconus. Eye & Contact Lens. 2014. (E-pub ahead of print).
43. Ferial MZ, Jawkhab AA, Al-Tuwairqi W, et al. Visual rehabilitation in low-moderate keratoconus: intracorneal ring segment implantation followed by same-day topography-guided photorefractive keratectomy and collagen cross linking. Int J Ophthalmol. 2014;7(5):800-6.
44. Chan CC, Sharma M, Wachler BS. Effect of inferior-segment Intacs with and without C3-R on keratoconus. J Cataract Refract Surg. 2007;33(1):75-80.
45. Kymionis GD, Kontadakis GA, Kounis GA, et al. Simultaneous topography-guided PRK followed by corneal collagen cross-linking for keratoconus. J Refract Surg. 2009;25(9):s807-11.
46. Kymionis GD, Karavitaki AE, Grentzelos MA, et al. Topography based keratoconus progression after corneal cross-linking. Cornea. 2014;33(4):419-21.
47. Keane M, Coster D, Ziaei M, et al. Deep anterior lamellar keratoplasty versus penetrating keratoplasty for treating keratoconus. Cochrane Database Syst Rev. 2014;22(7):CD009700.
48. Maccheron LJ, Daya SM. Wedge resection and lamellar dissection for pellucid marginal degeneration. Cornea. 2012;31(6):708-15.
49. Rasheed K, Rabinowitz YS. Surgical treatment of advanced pellucid marginal degeneration. Ophthalmol. 2000;107:1836-40.

