The Food and Drug Administration recently approved Galafold (migalastat, Amicus Therapeutics), the first oral medication to treat Fabry disease, an often-fatal genetic disorder with ocular manifestations. In fact, ocular signs of Fabry disease may be an early indicator that the condition exists in the patient’s genome.1
The most commonly reported ocular finding of the disease is corneal verticillata, a bilateral whorl-like pattern of radiating, cream-colored lines usually found in the inferior cornea.1 The deposits can be light or more pronounced and are located at the level of Bowman’s membrane.1-3
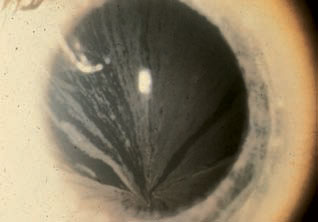 |
| Cornea verticillata in Fabry disease. Photo: R. L. Abbott, MD. |
A hallmark of Fabry disease is the buildup of globotriaosylceramide (GL-3), a type of fat found in blood vessels, kidneys, heart, nerves and other organs.
Traditionally, treatment was based on replacing the missing enzyme that causes the fat buildup.4 The new drug differs from enzyme replacement since it increases the activity of the body’s deficient enzyme that causes the GL-3 buildup.4
Fabry disease is an inherited disorder caused by mutations in the alpha-galactosidase A (GLA) gene located on the x-chromosome. The condition is rare and affects both males and females. Patients with this condition can develop kidney disease, cardiac hypertrophy, arrhythmias and stroke. Fabry disease can also result in early death.4
Researchers demonstrated the efficacy of Galafold in a six-month, placebo-controlled clinical trial in 45 adults with the disease. Patients treated with Galafold had a greater reduction in GL-3 in blood vessels of the kidneys compared the placebo group. The safety of Galafold was studied in four clinical trials that included a total of 139 patients with the disease.4
Since ocular manifestations can be present at an early age—and before the symptoms of more serious conditions associated with Fabry disease develop—early diagnosis by an eye care provider may reduce the morbidity and mortality associated with this condition.1
1. Morier AM, Lewis R. Genetics in Eye Care. Rev Optom. 2013;150(6):68-75. |

