Inflammation plays a significant role in the etiopathogenesis of dry eye.1 It promotes ocular surface disruption and symptoms of irritation. It is accompanied by increased osmolarity of the tear film and inflammation of the ocular surface.1,2 Once investigators identified inflammation’s role in dry eye development, research could target treatment using anti-inflammatory agents that inhibit the expression of inflammatory mediators on the ocular surface. By doing so, these agents help restore a healthy tear film and reduce signs and symptoms of afflicted patients.
This article reviews the inflammatory process, how different anti-inflammatory drugs can disrupt that process and how to appropriately apply that knowledge in your clinic.
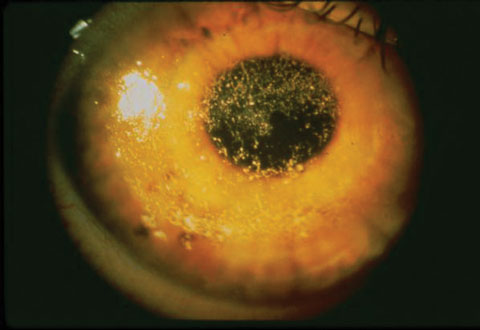 |
| This patient has severe dry eye due to Sjögren’s syndrome. Identifying the cause of a patient’s dry eye is key to targeting treatment. Photo: Robert Prouty, OD, Paul Ajamian, OD |
Pathophysiology
Growing evidence shows dry eye-related ocular surface inflammation is mediated by lymphocytes.3 Based on earlier immunohistopathological evaluations, patients with both Sjögren’s syndrome (SS) related and non-SS dry eye have identical conjunctival inflammation manifested by T-cell infiltrates and upregulation of CD3, CD4 and CD8, as well as lymphocyte activation markers CD11a and HLA-DR.4 These results suggested that clinical symptoms and signs of dry eye may be dependent on T-cell activation and resultant autoimmune inflammation. Multiple other studies demonstrated the role of proinflammatory cytokines and matrix metalloproteinases (MMPs) in the pathogenesis of dry eye.5-6
Interleukin (IL)-1 is one of the most widely studied cytokines accompanying dry eye. Researchers point to an increase in the proinflammatory forms of IL-1 (IL-1α and mature IL-1β) and a decrease in the biologically inactive precursor IL-1β found in the tear film of dry eye patients.5 Investigators recognize that IL-1β, IL-6, IL-8 and tumor necrosis factor (TNF)-α also play a significant role in SS-related dry eye as compared with healthy eyes.6 This explains why treatments in development today specifically target inflammatory cytokines.
The response of cells to extracellular stimuli, such as ocular surface stress due to changes in the tear film composition, hyperosmolarity or ultraviolet (UV) light exposure, is partially mediated by a number of intracellular kinase and phosphatase enzymes.7
According to one study, “mitogen-activated protein (MAP) kinases are integral components of parallel MAP kinase cascades activated in response to a number of cellular stresses including inflammatory cytokines (e.g., Il-1 and TNF-α), heat shock, bacterial endotoxin and ischemia.”7 Researchers have identified these stress-activated protein kinases in the tear film of patients with dry eye and documented that activation of the stress pathways results in the transcription of stress-related genes, including MMPs, mainly MMP-9.8
Another study shows that MAP kinases stimulate the production of inflammatory cytokines—including IL-β, TNF-α and MMP-9—causing ocular surface damage.9
Lymphocyte function-associated antigen-1 (LFA-1), with its cognate ligand intercellular adhesion molecule-1 (ICAM-1), plays an important role in the cell-mediated immune response and inflammation associated with dry eye.10 LFA-1 is expressed on the cell surface of leukocytes and binds with high affinity to ICAM-1 and with lower affinity to ICAM-2 and ICAM-3.11,12 ICAM-1 is expressed on the cell surface of leukocytes, endothelial cells, keratinocytes and epithelial cells.13 LFA-1 binding to ICAM-1 is involved in dendritic cell migration to regional lymph nodes in the afferent arm of the dry eye inflammatory pathway.14,15 LFA-1 and ICAM-1 may be involved in the dry eye immunoinflammatory efferent pathway as well.14-16
Naïve T-cells are primed in the lymph nodes through interaction with dendritic cells and differentiate to TH1 and TH17 effector cells.14-16
These activated CD4+ effector T-cells migrate from the lymph nodes to the ocular surface and lacrimal glands, where they exert inflammatory effects. Researchers suggest that LFA-1/ICAM-1 may play a role in reactivation of CD4+ cells at the ocular surface to further promote release of proinflammatory cytokines from either the T-cells or antigen presenting cells.17
Finally, research shows that inhibition of ICAM-1 and LFA-1 in mice reduces the number of inflammatory infiltrates in the lacrimal gland.18 Ultimately, it is possible that LFA-1/ICAM-1 may possibly recruit and retain LFA-1 expressing T-cells to the epithelium and conjunctiva, thus inducing proinflammatory cytokine release.
All these inflammatory mediators and pathways relate to the pathogenesis of dry eye and play a role in targeting treatment strategies.
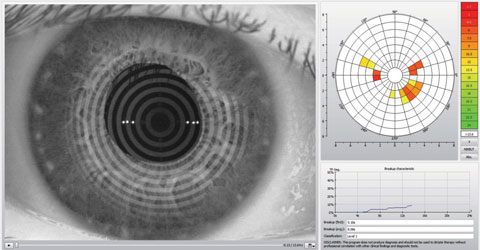 |
| This Keratograph 5M (Oculus) image shows an assessment of a patient’s noninvasive tear break-up time. Click image to enlarge. Photo: Dan Fuller, OD |
First-line Therapies
When treating dry eye, over-the-counter lubricants (e.g., artificial tears, gels, ointments) may be common but, for patients who need multiple doses per day or who have a punctal occlusion, your aim will be to reduce the cytotoxic effects often associated with those products, as they contain benzalkonium chloride (BAK). Instead, opt for preservative-free agents for these patients.
Before any other treatment, examine the patient for blepharitis. If present, the first step is to differentiate between bacterial and Demodex infections. In bacterial cases, it may be necessary to prescribe topical antibiotic or an antibiotic/steroid combination. If Demodex is at the root of the infection, turn to a product that contains 4-Terpineol, such as Cliradex (Biotissue) or Cliradex Light.
Corticosteroids
Topical steroids, through several mechanisms, help reduce ocular inflammation. Corticosteroids function via suppression of cellular infiltration, capillary dilation, proliferation of fibroblasts and collagen deposition.19 They stabilize intracellular and extracellular membranes.19
Corticosteroids increase the synthesis of lipocortins that block phospholipase A2 and inhibit histamine synthesis in the mast cells.19 Inhibition of phospholipase A2, an essential step in the inflammatory cascade, prevents the conversion of phospholipids to arachidonic acid. Corticosteroids also interfere with transcription factor NF-kB, which regulates synthesis of a number of proinflammatory molecules, thereby stimulating lymphocyte apoptosis.
Corticosteroids mediate their anti-inflammatory effects primarily through the modulation of the cytosolic glucocorticoid receptor at the genomic level.20,21 After corticosteroids bind to the glucocorticoid receptor in the cytoplasm, the activated corticosteroid-glucocorticoid receptor complex migrates to the nucleus, where it upregulates the expression of anti-inflammatory proteins and represses the expression of proinflammatory proteins.20,21 However, recent work suggests the activated corticosteroid-glucocorticoid receptor complex also elicits nongenomic effects, such as inhibition of vasodilation, vascular permeability and migration of leukocytes.20,22
Several clinical studies demonstrate the effectiveness of topical steroids in treating dry eye.23-25 In a retrospective clinical series, topical administration of a 1% solution of nonpreserved methylprednisolone, given TID or QID for several weeks to patients with SS-related dry eye, provided moderate to complete relief of symptoms in all patients.23 In addition, a decrease in corneal fluorescein staining score (2.6 ± 0.5 on a 12-point scale) and complete resolution of filamentary keratitis were seen.23 This therapy was effective even for patients suffering from severe dry eye who had no improvement from maximum aqueous tear enhancement/replacement therapies.23
One pilot study looked at 64 patients to evaluate the efficacy of Lotemax (loteprednol etabonate 0.5%, Bausch + Lomb) ophthalmic suspension QID vs. placebo to treat the inflammatory component of dry eye associated with aqueous tear deficiency and delayed tear clearance.24 After two weeks of therapy, Lotemax-treated patients with moderate to severe clinical inflammation showed a significant decrease in central corneal staining, nasal bulbar conjunctival hyperemia and lid margin injection, compared with the placebo group.24 No patients experienced clinically significant increase in intraocular pressure following one month of therapy.24
Patients treated with topical corticosteroids should be monitored closely for known risks of cataract formation, glaucoma, corneal thinning and infectious keratitis.25
NSAIDs
Topical nonsteroidal anti-inflammatory drugs (NSAIDs) are used to manage allergic conjunctivitis, postoperative ocular pain, cystoid macular edema after cataract surgery and several other conditions in addition to dry eye. NSAIDs treat inflammation by inhibiting the production of prostaglandins via the cyclooxygenase enzyme.26 However, research shows NSAIDs—specifically diclo-fenac—can reduce corneal sensitivity, too.27 This may cause insult to the disrupted epithelium in dry eye patients.28-34 The literature shows several cases of corneal melt associated with use of topical NSAIDs, including diclofenac, ketorolac, nepafenac and bromfenac.28-34 In all of these cases, preexisting epitheliopathy was identified.28-34 Although the exact relationship between corneal melt and topical NSAID use is still not clear, various suggested mechanisms include activation of matrix metalloproteinases, impairment of wound healing and neurotrophic effect resulting from the analgesic action of these drugs.28-34
Short-term use of NSAIDs can be useful in ameliorating symptoms of ocular discomfort in dry eye. However, they should be used with caution and under close monitoring, and the treatment should be preferably discontinued if the corneal epithelium becomes damaged.
Cyclosporin A
The immunomodulating effects of cyclosporin A are achieved through binding with cyclophilins. Cyclophilin A, which is found in the cytosol, and the cyclosporin-cyclophilin A complex inhibits a calcium/calmodulin-dependent phosphatase, calcineurin, the inhibition of which is thought to halt the production of the transcription of T-cell activation by inhibiting IL-2.35
Cyclophilin D is located in the matrix of mitochondria. Cyclosporin A-cyclophilin D complex modulates the mitochondrial permeability transition pore, thereby inducing a mitochondrial dysfunction and cell death.36 The reduction in inflammation, via inhibition of T-cell activation and downregulation of inflammatory cytokines in the conjunctiva and lacrimal gland, allows enhanced tear production.37-41 Topical cyclosporine also increases goblet cell density and decreases epithelial cell apoptosis.42 Commercially available Restasis (topical cyclosporine 0.05%, Allergan) or a 1% compounded preparation is frequently used to treat various inflammatory ocular surface disorders.43
Dosing topical cyclosporine at a frequency greater than twice a day may be more effective for patients who do not demonstrate improvement of severe dry eye disease with the twice-daily regimen.44,45
Lifitegrast
This formulation blocks the binding of the surface proteins LFA-1 and ICAM-1, thereby reducing inflammation in dry eye.46 The recommended dosing of the commercially available Xiidra (lifitegrast 5%, Shire) is twice daily.47 Researchers recently completed a one-year multicenter, randomized, placebo-controlled study of the safety of lifitegrast ophthalmic solution 5.0% in 331 participants (220 lifitegrast, 111 placebo) with dry eye.47 There were no serious treatment-emergent adverse events (TEAEs). Overall, 53.6% of participants receiving lifitegrast experienced ≥1 ocular TEAEs vs. 34.2% in the placebo group.47 Most TEAEs were mild-moderate in severity. Rates of discontinuation because of TEAEs were 12.3% (lifitegrast) vs. 9.0% (placebo).47 The most common (>5%) TEAEs occurring in either treatment group were instillation site irritation (burning), instillation site reaction, reduced visual acuity, dry eye and dysgeusia (change in taste).47 There was no indication of systemic toxicity or localized infectious complications secondary to chronic immunosuppression.
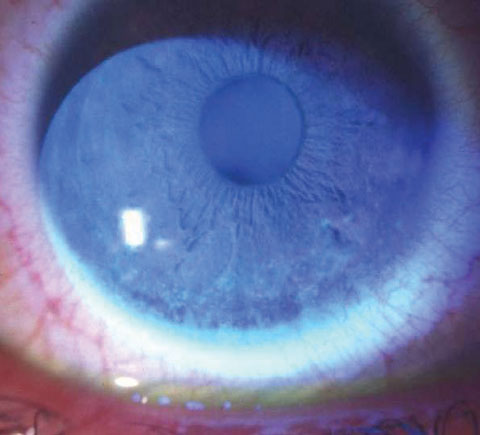 |
| Superficial punctate keratitis in a Sjögren’s patient on Restasis therapy. Photo: Robert Prouty, OD, Paul Ajamian, OD |
Tacrolimus
This topical anti-inflammatory agent (previously known as FK506) is a macrolide antibiotic.48 Although the mechanism of tacrolimus is similar to cyclosporin A, research shows the potency in vitro has been shown to be significantly greater.49 Only when bound to immunophilin does it become biologically active, thus effectively inhibiting calcineurin, and inhibiting T- and B-lymphocyte activation via reduction in IL-2 synthesis.48,50,51 Tacrolimus suppresses the immune response by inhibiting the release of other inflammatory cytokines as well, such as IL-4 and IL-8.50,52,53
Systemic tacrolimus has been reported to be effective for improving dry eye associated with graft vs. host disease (GVHD). However, there are potential adverse reactions to be aware of when administering long-term systemic therapy.54 Topical tacrolimus is available in 0.03% and 0.1% concentrations as an ointment (typically applied externally to eyelids) as well as compounded eye drops. It is a promising off-label treatment of dry eye in the setting of chronic GVHD and SS.55-57
Tetracycline Derivatives
Oral tetracycline derivatives possess antibacterial as well as anti-inflammatory properties. Doxycycline has been shown to inhibit c-Jun N-terminal kinase and extracellular signal-related kinase mitogen-activated protein kinase signaling in epithelial cells of the ocular surface exposed to hyperosmolar stress, downregulating the expression of CXCL8 and proinflammatory cytokines IL-1β and TNF.58 Doxycycline inhibits MMP-9 activity and supports ocular surface integrity.59,60
Additionally studies demonstrated that minocycline inhibits expression of cell-associated proinflammatory molecules, including major histocompatibility complex class II.61 Doxycycline has been reported to be effective in patients with ocular rosacea by reducing irritation symptoms, improving tear film stability and decreasing the severity of ocular surface disease.62-64 In addition, doxycycline has been useful in the treatment of corneal erosions.65,66
Azithromycin
This broad-spectrum macrolide antibiotic has been shown to have good tissue penetration to the eyelid and favorable pharmacokinetics for daily dosing. Azasite (topical azithromycin, Akorn) is FDA approved to treat bacterial conjunctivitis, but may be used as off-label therapy for clinical control or relief of symptoms and signs of meibomian gland dysfunction, as well as improvement in lipid behaviors of meibomian gland secretion.67 It has also been noted that topical azithromycin management could lead to improvement in meibomian gland orifice plugging.68 A single oral dose of 1g has been shown to provide prolonged high levels after 14 days in drug-targeted ocular tissues, to decrease inflammatory cytokines and suppress production of proinflammatory mediators.69-72 Good intracellular penetration and long half-life of azithromycin can provide an effective antimicrobial and favorable immunomodulatory effect without compliance issues of long-term tetracycline use.70,72 Research shows the drug could block activation of NF-kB, leading to decreased inflammatory cytokine levels such as IL-6 and IL-8.73 Besides, azithromycin has been shown to suppress the production of proinflammatory mediators by inhibiting cultured human corneal epithelial cells.74
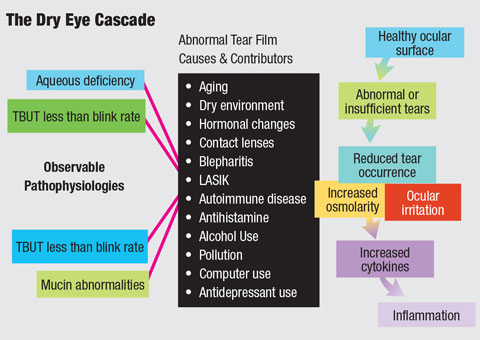 |
| The increased prevalence of dry eye disease can be attributed to a number of factors. Understanding the mechanism of action behind therapeutic options can help you best target your patients’ treatments. Diagram: Bruce Onofrey, OD, RPh |
Autologous serum
Serum contains several anti-inflammatory factors that have the capability to inhibit soluble mediators of the ocular surface inflammatory cascade of dry eye. These include inhibitors of inflammatory cytokines (e.g., IL-1 RA and soluble TNF-receptors) and MMP inhibitors (e.g., tissue inhibitors of metalloproteinases).75-77 Clinical trials show autologous serum drops improve ocular irritation symptoms and conjunctival and corneal dye staining in dry eye that occurs in the setting of SS.78-80 Conversely, there is greater risk of microbial growth as autologous serum drops, in addition to antimicrobial agents, contain high protein content and are generally nonpreserved.81
Recent studies have investigated cord serum drops (prepared from donor umbilical cord serum) as well as allogenic serum drops (from a relative donor).82-84 A clinical trial of 17 patients with GVHD and 13 patients with SS-associated dry eye treated them for one month with cord blood serum. Patients received cord blood once a day (containing 0.15ng epithelial growth factor per drop). Patients reported a decrease in discomfort symptoms as measured with the Ocular Surface Disease Index score (OSDI) (22.3 ± 10.3 vs. 39.3 ± 16.9). Also, clinical findings such as impression cytology score (3.8 ± 1.2 vs. 6.6 ± 2.1), tear osmolarity (312.5 ± 7 vs. 322 ± 9.1mOsm/L), and corneal sensation (measured with Cochet-Bonnet esthesiometers) (48.2 ± 2.1 vs. 49.7 ± 2.1 nylon/mm/length) improved significantly.82
Another study, this one involving 12 patients with chronic GVHD-associated severe dry eye treated with cord blood serum for six months, reported statistically significant improvement (p<0.01) in symptom score (on a scale of 0-4, 3.83+/-0.38 vs. 0.83+/-0.57), corneal sensitivity (52.08+/-6.06mm to 57.50+/-3.00mm), tear film BUT (from 2.50+/-0.91 sec. to 5.71+/-1.04 sec., P<0.01) and corneal fluorescein staining (7.42+/-2.02 to 1.29+/-0.46).83
Allogenic serum drops are prepared using blood from a family member rather than the patient’s own. In one study, allogeneic serum tears were used for the treatment of dry eye in 16 patients with GVHD. After four weeks of continuous use the symptom scores (32.5-8.9 OSDI score), tear osmolarity (311.1 to 285.1mOsmL), and corneal staining (2.5 to 1.8) improved as well as increased goblet cell density (90.6 to 122.6 cell/mm2), and tear break-up time (2.9 to 4.4 sec.).84
Dry eye therapy should target the inflammatory cascade, given its significant role in etiopathogenesis. When selecting an appropriate treatment plan, it is important to consider severity of the condition based on clinical exam including osmolarity, Schirmer, tear break-up time and ocular surface staining. It is also necessary to identify concurrent ocular disease as well as possible systemic conditions that may be contributing factors. The treatment goal is to improve patient symptoms, restore a healthy tear film, prevent further destruction of the ocular surface and ultimately reestablish an intact epithelium.
Dr. Hessen is a clinical instructor at the Wilmer Eye Institute’s Ocular Surface Diseases and Dry Eye Clinic at Johns Hopkins School of Medicine, where she specializes in ocular surface disease, including autoimmune disorders.
| 1. Hessen M, Akpek E. Dry eye: an inflammatory ocular disease. J Ophthalmic Vis Res. 2014;9(2):240–50. 2. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5(2):75-92. 3. Stern ME, Schaumburg CS, Pflugfelder SC. Dry eye as a mucosal autoimmune disease. Int Rev Immunol. 2013;32(1):19-41. 4. Stern M, Gao J, Schwalb T, et al. Conjunctival T-cell subpopulations in Sjögren’s and non-Sjögren’s patients with dry eye. Invest Ophthalmol Vis Sci. 2002;43:2609–14. 5. Solomon A, Dursun D, Liu Z, et al. Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci. 2001;42:2283–92. 6. Cejková J, Ardan T, Simonová Z, et al. Nitric oxide synthase induction and cytotoxic nitrogen-related oxidant formation in conjunctival epithelium of dry eye (Sjögren’s syndrome). Nitric Oxide. 2007;17:10–7. 7. Paul A, Wilson S, Belham C, et al. Stress-activated protein kinases: activation, regulation and function. Cell Signal. 1997;9:403–10. 8. Pflugfelder S, de Paiva C, Tong L, et al. Stress-activated protein kinase signaling pathways in dry eye and ocular surface disease. Ocul Surf. 2005;3(Suppl 4):154–7. 9. Luo L, Li D, Doshi A, et al. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci. 2004;45:4293–301. 10. Pflugfelder S, Stern M, Zhang S, Shojaei A. LFA-1/ICAM-1 interaction as a therapeutic target in dry eye disease. J Ocul Pharmacol Ther. 2017;33(1):5-12. 11. Evans R, Patzak I, Svensson L, et al. Integrins in immunity. J Cell Sci. 2009;122:215–25. 12. de Fougerolles A, Springer T. Intercellular adhesion molecule 3, a third adhesion counter-receptor for lymphocyte function-associated molecule 1 on resting lymphocytes. J Exp Med. 1992;175:185–190. 13. Roebuck K, Finnegan A. Regulation of intercellular adhesion molecule-1 (CD54) gene expression. J Leukoc Biol. 1999;66:876–88. 14. Stern M, Schaumburg C, Dana R, et al. Autoimmunity at the ocular surface: pathogenesis and regulation. Mucosal Immunol. 2010;3:425–42. 15. Stern M, Schaumburg C, Pflugfelder S. Dry eye as a mucosal autoimmune disease. Int Rev Immunol. 2013;32:19–41. 16. Perez V, Pflugfelder S, Zhang S, et al. A novel integrin antagonist for treatment of dry eye disease. Ocul Surf. 2016;14:207–15. 17. Schaumburg C, Siemasko K, De Paiva C, et al. Ocular surface APCs are necessary for autoreactive T cell-mediated experimental autoimmune lacrimal keratoconjunctivitis. J. Immunol. 2011;187:3653–62. 18. Gao J, Morgan G, Tieu D, et al. ICAM-1 expression predisposes ocular tissues to immune-based inflammation in dry eye patients and Sjögrens syndrome-like MRL/lpr mice. Exp Eye Res. 2004;78:823–35. 19. Comstock T, DeCory H. Advances in corticosteroid therapy for ocular inflammation: loteprednol etabonate. Int J Inflam. 2012;2012:789623. 20. Rhen T, Cidlowski J. Antiinflammatory action of glucocorticoids - new mechanisms for old drugs. N Engl J Med. 2005;353:1711–23. 21. Newton R. Molecular mechanisms of glucocorticoid action: what is important? Thorax. 2000;55:603–13. 22. Stahn C, Buttgereit F. Genomic and nongenomic effects of glucocorticoids. Nat Clin Pract Rheumatol. 2008;4:525–33. 23. Marsh P, Pflugfelder S. Topical nonpreserved methylpresdnisolone therapy of keratoconjunctivits sicca in sjogren’s syndrome. Ophthalmology. 1999;106:811–16. 24. Pflugfelder S, Maskin S, Anderson B, et al. A randomized, double-masked, placebo-controlled, multicenter comparison of loteprednol etabonate ophthalmic suspension, 0.5%, and placebo for treatment of keratoconjunctivitis sicca in patients with delayed tear clearance. Am J Ophthalmol. 2004;138:444–57. 25. McGhee C, Dean S, Danesh-Meyer H. Locally administered ocular corticosteroids: benefits and risks. Drug Saf. 2002;25:33–55. 26. Vane J, Bakhle Y, Botting R. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. 27. Aragona P, Tripodi G, Spinella R, et al. The effects of the topical administration of non-steroidal anti-inflammatory drugs on corneal epithelium and corneal sensitivity in normal subjects. Eye. 2000;14(2):206-10. 28. Gokhale NS, Vemuganti GK. Diclofenac-induced acute corneal melt after collagen crosslinking for keratoconus. Cornea. 2010;29:117–9. 29. Flach A. Corneal melts associated with topically applied nonsteroidal anti-inflammatory drugs. Trans Am Ophthalmol Soc. 2001;99:205–12. 30. Khalifa Y, Mifflin M. Keratitis and corneal melt with ketorolac tromethamine after conductive keratoplasty. Cornea. 2011;30:477–8. 31. di Pascuale M, Whitson J, Mootha V. Corneal melting after use of nepafenac in a patient with chronic cystoid macular edema after cataract surgery. Eye Contact Lens. 2008;34:129–30. 32. Asai T, Nakagami T, Mochizuki M, et al. Three cases of corneal melting after instillation of a new nonsteroidal anti-inflammatory drug. Cornea. 2006;25:224–7. 33. Isawi H, Dhaliwal D. Corneal melting and perforation in Stevens Johnson syndrome following topical bromfenac use. J Cataract Refract Surg. 2007;33:1644–6. 34. Prasher P. Acute corneal melt associated with topical bromfenac use. Eye Contact Lens. 2012;38:260–2. 35. Matsuda S, Koyasu S. Mechanisms of action of cyclosporine. Immunopharmacology. 2000;47:119–25. 36. Stevenson W, Chauhan SK, Dana R. Dry Eye Disease: an immune-mediated ocular surface disorder. Arch Ophthalmol. 2012;130:90–100. 37. Pflugfelder S, Wilhelmus K, Osato M, et al. The auto-immune nature of aqueous tear deficiency. Ophthalmol. 1986;93:1513–7. 38. Stern M, Gao J, Siemasko K, et al. The role of the lacrimal gland functional unit in the pathophysiology of dry eye. Exp Eye Res. 2004;78:409–16. 39. Stevenson D, Tauber J, Reis BL. Efficacy and safety of cyclosporine A ophthalmic emulsion in the treatment of moderate to severe dry eye disease: a dose-ranging, randomized trial. The Cyclospoine A Phase 2 Study Group. Ophthalmol. 2000;107:967–74. 40. Sall K, Stevenson O, Mundorf T, Reis B. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmol. 2000;107:631–9. 41. Laibovitz R, Solch S, Andriano K, et al. Pilot trial of cyclosporine 1% ophthalmic ointment in the treatment of keratoconjunctivitis sicca. Cornea. 1993;12:315–23. 42. Kunert K, Tisdale A, Gipson I. Goblet cell numbers and epithelial proliferation in the conjunctiva of patients with dry eye syndrome treated with cyclosporine. Arch Ophthalmol. 2002;120:330–7. 43. Utine C, Stern M, Akpek E. Clinical review: topical ophthalmic use of cyclosporin A. Ocul Immunol Inflamm. 2010;18:352–61. 44. Gupta A, Sadeghi P, Akpek E. Occult thyroid eye disease in patients presenting with dry eye symptoms. Am J Ophthal. 2009;147:919–23. 45. Dastjerdi M, Hamrah P, Dana R. High-frequency topical cyclosporine 0.05% in the treatment of severe dry eye refractory to twice-daily regimen. Cornea. 2009;28:1091–6. 46. American Academy of Ophthalmology Cornea/External Disease PPP Panel, Hoskins Center for Quality Eye Care. www.aao.org/preferred-practice-pattern/dry-eye-syndrome-ppp–2013. October 2013. Accessed March 22, 2017. 47. Donnenfeld E, Karpecki P, Majmudar P, et al. Safety of Lifitegrast ophthalmic solution 5.0% in patients with dry eye disease: A 1-year, multicenter, randomized, placebo-controlled study. Cornea. 2016;35(6):741–8. 48. Thomson A, Bonham C, Zeevi A. Mode of action of tacrolimus (FK506): molecular and cellular mechanisms. Ther Drug Monit. 1995;17:584–91. 49. Kino T, Hatanaka H, Hashimoto M, et al. FK-506, a novel immunosuppressant isolated from Streptomyces. I. Fermentation isolation, and physio-chemical and biological characteristics. J Antibiot (Tokyo). 1987;40:1249–55. 50. Fei W, Chen J, Yuan J, et al. Preliminary study of the effect of FK506 nanospheric-suspension eye drops on rejection of penetrating keratoplasty. J Ocul Pharmacol Ther. 2008;24:235–44. 51. Fujita E, Teramura Y, Mitsugi K, et al. Absorption, distribution, and excretion of 14C-labeled tacrolimus (FK506) after a single or repeated ocular instillation in rabbits. J Ocul Pharmacol Ther. 2008;24:333–43. 52. Nishino K, Fukushima A, Okamoto S, et al. Suppression of experimental immune-mediated blepharoconjunctivitis in brown Norway rats by topical application of FK506. Graefes Arch Clin Exp Ophthalmol. 2002;240:137–43. 53. Sasakawa Y, Sakuma S, Higashi Y, et al. FK506 suppresses neutrophil chemoattractant production by peripheral blood mononuclear cells. Eur J Pharmacol. 2000;403:281–8. 54. Aoki S, Mizote H, Minamoto A, et al. Systemic FK506 improved tear secretion in dry eye associated with chronic graft versus host disease. Br J Ophthalmol. 2005;89:243–4. 55. Ryu E, Kim J, Laddha P, et al. Therapeutic effect of 0.03% tacrolimus for ocular graft versus host disease and vernal keratoconjunctivitis. Korean J Ophthalmol. 2012;26:241–7. 56. Tam P, Young A, Cheng A, Lam P. Topical 0.03% tacrolimus ointment in the management of ocular surface inflammation in chronic GVHD. Bone Marrow Transplant. 2010;45:957–8. 57. Moscovici B, Holzchuh R, Chiacchio B, et al. Clinical treatment of dry eye using 0.03% tacrolimus eye drops. Cornea. 2012;31:945–9. 58. Solomon A, Rosenblatt M, Li D, et al. Doxycycline inhibition of interleukin-1 in the cornea epithelium. Invest Ophthalmol Vis Sci. 2000 Aug;41(9):2544-57. 59. De Paiva C, Corrales R, Villarreal A, et al. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Exp Eye Res. 2006;83:526–35. 60. De Paiva C, Corrales R, Villarreal A, et al. Apical corneal barrier disruption in experimental murine dry eye is abrogated by methylprednisolone and doxycycline. Invest Ophthalmol Vic Sci. 2006;47:2847–56. 61. Nikodemova M, Watters J, Jackson S, et al. Minocycline downregulates MHC II expression in microglia and macrophages through inhibition of IRF-1 and protein kinase C (PKC) alpha/betaII. J Biol Chem. 2007;282:15208–16. 62. Frucht-Pery J, Sagi E, Hemo I, Ever-Hadani P. Efficacy of doxycycline and tetracycline in ocular rosacea. Am J Ophthalmol. 1993;116:88–92. 63. Zengin N, Tol H, Gündüz K, et al. Meibomian gland dysfunction and tear film abnormalities in rosacea. Cornea. 1995;13:144–14. 64. Akpek E, Merchant A, Pinar V, Foster C. Ocular rosacea: patient characteristics and follow-up. Ophthalmol. 1997;104:1863–7. 65. Dursun D, Kim M, Solomon A, Pflugfelder S. Treatment of recalcitrant corneal epithelial erosions with inhibitors of matrix metalloproteinases-9, doxycycline and corticosteroids. Am J Ophthalmol. 2001;132:8–13. 66. Hope-Ross MW, Chell PB, Kervick GN, et al. Oral tetracycline in the treatment of recurrent corneal erosions. Eye (Lond). 1994;8:384–88. 67. Foulks GN, Borchman D, Yappert M, et al. Topical azithromycin therapy for meibomian gland dysfunction: clinical response and lipid alterations. Cornea. 2010;29(7):781–8. 68. Haque R, Torkildsen G, Brubaker K, et al. Multicenter open-label study evaluating the efficacy of azithromycin ophthalmic solution 1% on the signs and symptoms of subjects with blepharitis. Cornea. 2010;29(8):871–7. 69. Qiao J, Yan X. Emerging treatment options for meibomian gland dysfunction. Clin Ophthalmol. 2013;7:1797–803. 70. Kashkouli M, Fazel A, Kiavash V, et al. Oral azithromycin versus doxycycline in meibomian gland dysfunction: a randomised double-masked open-label clinical trial. Br J Ophthalmol. 2015;99:199–204. 71. Greene J, Jeng B, Fintelmann R, Margolis T. Oral azithromycin for the treatment of meibomitis. JAMA Ophthalmol. 2014;132:121–2. 72. Igami T, Holzchuh R, Osaki TH, et al. Oral azithromycin for treatment of posterior blepharitis. Cornea. 2011;30:1145–9. 73. Aghai Z, Kode A, Saslow J, et al. Azithromycin suppresses activation of nuclear factor-kappa B and synthesis of pro-inflammatory cytokines in tracheal aspirate cells from premature infants. Pediatr Res. 2007;62(4):483–8. 74. Li D, Zhou N, Zhang L, et al. Suppressive effects of azithromycin on zymosan-induced production of proinflammatory mediators by human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2010;51(11):5623–9. 75. Liou L. Serum and in vitro production of IL-1 receptor antagonist correlate with C-reactive protein levels in newly diagnosed, untreated lupus patients. Clin Exp Rheumatol. 2001;19:515–23. 76. Ji H, Pettit A, Ohmura K, et al. Critical roles for interleukin 1 and tumor necrosis factor alpha in antibody-induced arthritis. J Exp Med. 2002;196:77–85. 77. Paramo J, Orbe J, Fernandez J. Fibrinolysis/proteolysis balance instable angina pectoris in relation to angiographic findings. Thromb Haemost. 2001;86:636–9. 78. Fox R, Chan R, Michelson J, et al. Beneficial effect on artificial tears made with autologous serum in patients with Keratoconjunctivitis sicca. Arthritis Rheum. 1984;27:459–61. 79. Kono I, Kono K, Narushima K, et al. Beneficial effect of the local application of plasma fibronectin and autologous serum in patients with Keratoconjunctivitis sicca of Sjogren’s syndrome. Ryumachi. 1986;26:339–43. 80. Tsubota K, Goto E, Fujita H, et al. Treatment of dry eye by autologous serum application in Sjogren’s syndrome. Br J Ophthamol. 1999;83:390–5. 81. Tananuvat N, Daniell M, Sullivan L, et al. Controlled study of the use of autologous serum in dry eye patients. Cornea. 2001;20:802–6. 82. Versura P, Profazio V, Buzzi M, et al. Efficacy in standardized and quality-controlled cord blood serum eye drop therapy in the healing of severe corneal epithelial damage in dry eye. Cornea. 2013;32:412–8. 83. Yoon K, Jeong I, Im S, et al. Therapeutic effect of umbilical cord serum for the treatment of dry eye associated with graft-versus-host disease. Bone Marrow Transplant. 2007;39:231–5. 84. Na K, Kim M. Allogenic serum eye drops for the treatment of dry eye patients with chronic graft-versus-host disease. J Ocul Pharmacol Ther. 2012;28:479–83. 1. |

