Managing ocular surface disease (OSD) is crucial for contact lens success. Contact lens dropout rates remain as high as 64% when symptoms such as discomfort and dry eye disease (DED) are present.1 Ensuring a healthy ocular surface prior to your contact lens fitting can minimize the chance of contact lens issues down the road and increase patient satisfaction.
 |
|
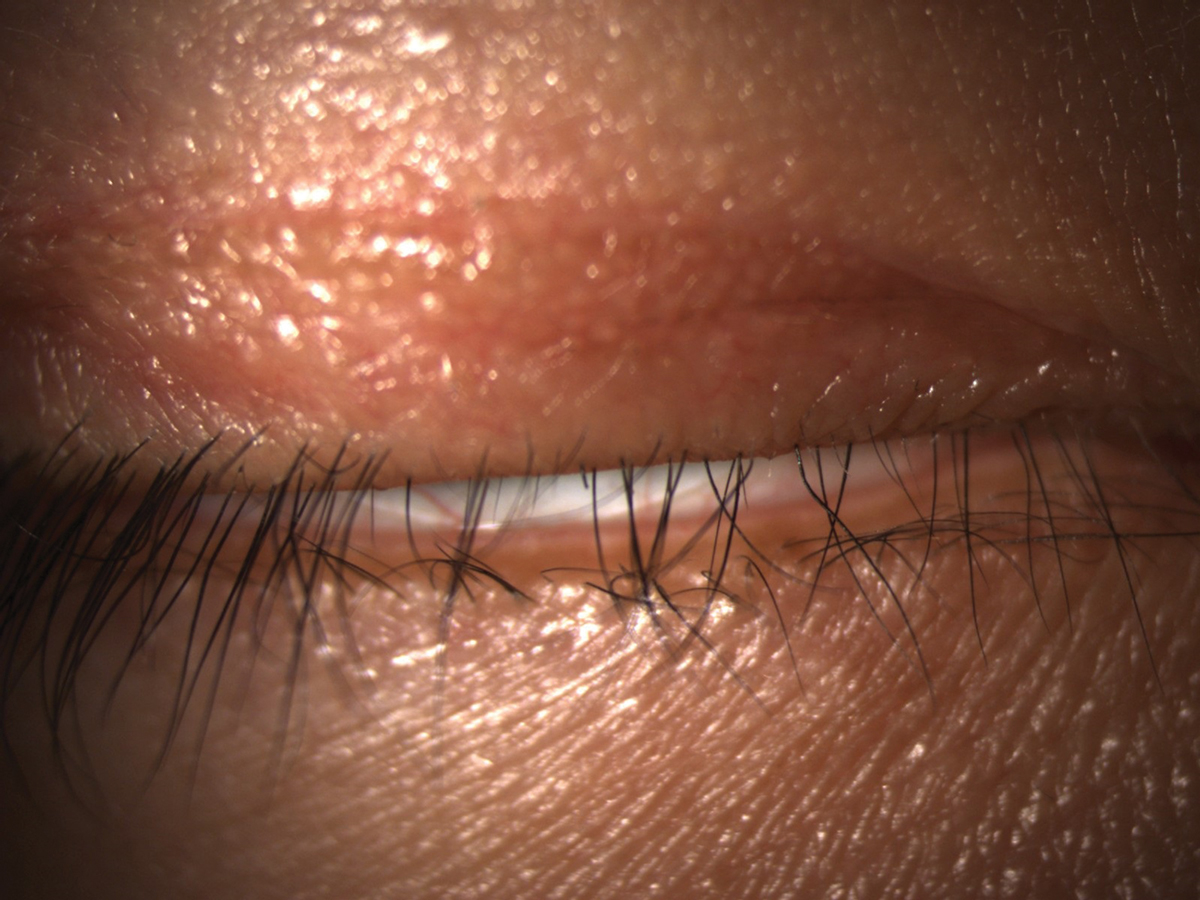
Lagophthalmos can cause inferior corneal exposure and contribute to DED. Click image to enlarge. |
Baseline Evaluation
DED is a complex, multifactorial condition where symptoms and clinical signs do not always correlate well. Patients may be asymptomatic or could complain of pain, tearing or blurry vision. Many have found symptoms to be a more reliable indicator of dry eye than clinical signs.2 As such, incorporate a validated dry eye questionnaire such as SPEED (Standard Patient Evaluation of Eye Dryness) or OSDI (Ocular Surface Disease Index) in your patient work-up. The SPEED questionnaire has the advantages of fewer questions and easier interpretation. Contact lens dropouts have been found to have significantly higher scores on SPEED than successful lens wearers.1,3
Always consider the patient’s medical history and medications while caring for their ocular health. Medical conditions that may contribute to DED include Sjögren’s syndrome, rheumatoid arthritis, lupus and thyroid disorders amongst many others. The Tear Film and Ocular Surface Society’s Dry Eye Workshop (DEWS) II report includes an extensive list of medications that can contribute to DED. Common classes of such medications include antihistamines, antidepressants, antispychotics, estrogen replacements and decongestants.4 Chronic use of topical preservatives may also contribute to DED. Be aware of this in glaucoma and ocular hypertensive patients, as many of them are on such topical medications for many years.
Table 1. Normal vs. Abnormal Test Levels for OSD | ||
| Normal Value | Abnormal Value | |
| Osmolarity | <300mOsm/L | > 300mOsm/L |
MMP-9 levels | <40ng/mL | >40ng/mL |
Phenol red | >20mm | <20mm |
Schirmer’s without anesthetic | >10mm | <10mm |
Perform a careful slit lamp examination prior to initiating any contact lens fit. Eyelid position, closure and blink rate should be observed. Evaluate eyelids and lashes for signs of blepharitis or meibomian gland dysfunction (MGD).
Signs of blepharitis include inflamed lid margins and debris along the lashes. Anterior blepharitis is found on the outer edge of the lid margin and is often a result of a bacterial infection. Posterior blepharitis is found on the inner edge and involves the meibomian glands. This is a critical step in your baseline evaluation because MGD is the major cause of evaporative dry eye and the odds of contact lens dropout increase with worsening levels of MGD.1 Additionally, numerous studies demonstrate that contact lens wear negatively affects the meibomian glands, causing increased levels of gland dropout and lower-quality meibum.5
Evaluate the conjunctiva and eyelid margin with lissamine green staining. This vital dye stains epithelial cells with damaged cell membranes and is useful in diagnosing early to moderate DED. Nine or more spots of lissamine staining are considered clinically significant.4 Lissamine can also be used to examine the upper and lower lid margins to look for signs of lid wiper epitheliopathy. This staining represents increased friction that occurs when the lid margins contact an ocular surface with a thin or unstable tear film.4 Examine the cornea with both white light and a cobalt blue filter with fluorescein, looking for subtle changes like punctate epithelial defects. Fluorescein will stain compromised cells, and five or more spots of staining are considered significant.4 Remember to wait at least one minute after instilling these dyes for a more accurate assessment.
Fluorescein can also be used to assess tear break-up time (TBUT), but the sensitivity and specificity of the TBUT is less certain for patients with milder cases of DED.4 Consider performing several TBUT measurements and averaging your results for greater accuracy.
Differential DiagnosesOther conditions such as allergic conjunctivitis, viral conjunctivitis and Demodex can present with symptoms of redness and tearing that mimic dry eye. Clinical findings that point to an allergic etiology include conjunctival papillae or chemosis and eyelid edema. Itching is a hallmark symptom of allergic conjunctivitis. Key signs of viral conjunctivitis include watery discharge, edematous lids and preauricular lymphadenopathy. In viral infections, redness often begins unilaterally and then affects the other eye within a few days. Key signs of viral conjunctivitis include watery discharge, edematous lids and preauricular lymphadenopathy. Also consider Demodex in your differentials, as these mites can spread to the eyelids. During a slit lamp exam, cylindrical dandruff at the root of the eyelash is a key finding that suggests Demodex. The mites may also be viewed under high magnification after epilating a lash. |
If you have access to a TearLab device, consider obtaining tear film osmolarity. Hyperosmolarity is associated with proinflammatory stress that contributes to DED severity. A positive result is greater than 300mOsm/L or an interocular difference greater than 8mOsm/L. A study looking at variability of indicators, including corneal staining, conjunctival staining, meibomian grading, TBUT, Schirmer tests and OSDI, found that the osmolarity measurement was least variable in patients with dry eye.6
Tear hyperosmolarity initiates an inflammatory cascade that eventually causes damage to the ocular surface. Matrix metalloproteinase-9 (MMP-9) is one of many inflammatory markers that increases with increased levels of dryness. The InflammaDry (Quidel) device can measure MMP-9 levels, and levels 40ng/ml or greater are significant and produce a positive result.4
Also consider using the Schirmer or phenol red tests. The phenol red test involves inserting a thin yellow string into the eye for 15 seconds that turns red when moistened by tears. A normal result is greater than 20mm. Eyes are considered dry with a result less than 10mm and marginally dry with a result between 10mm and 20mm. Phenol red testing is less likely to cause reflex tearing than the Schirmer’s test, which consists of a paper strip that is placed on the eye for five minutes. For Schirmer’s testing without anesthetic, results less than 5mm are considered dry and between 5mm to 10mm are marginally dry. There is conflicting data on the sensitivity and specificity of these tests as well as the correlation between their results.4 All of these testing values are provided (Table 1).
Baseline imaging will allow you to better monitor your patients with OSD over time and ensure that contact lens wear is not exacerbating any anterior segment findings. Anterior segment photos allow you to document existing corneal or conjunctival staining, corneal neovascularization or scarring. In moderate-to-severe OSD, you may also use topography to rule out the presence of irregular astigmatism. A device such as the LipiView (TearScience) or Keratograph (Oculus) can quantify additional indicators of DED such as lipid layer thickness and tear meniscus height.
DED is complex, and there is no one test to diagnose it. Consider all the diagnostic tools available as well as the patient’s symptoms to formulate the best diagnosis and management plan.
 |
|
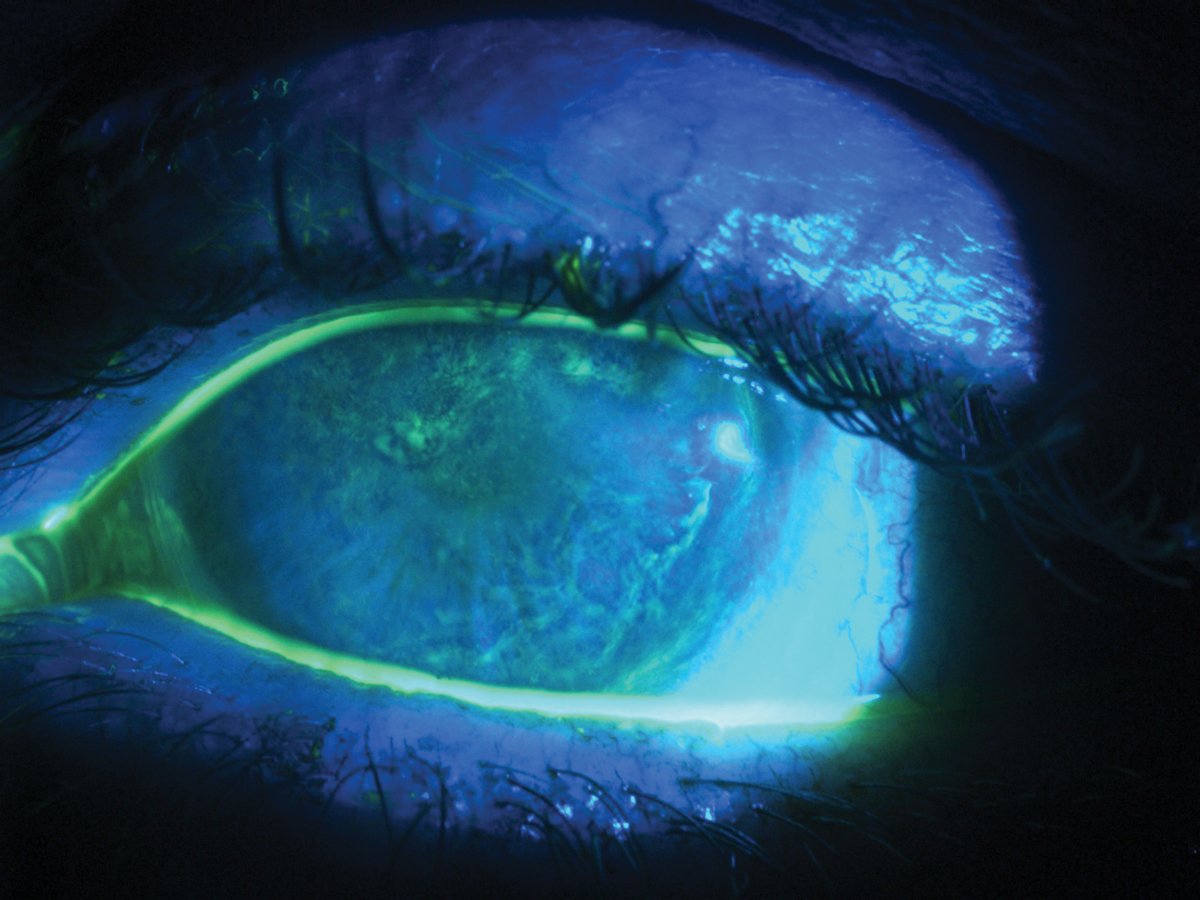
Severe OSD may present as diffuse, whorl-like staining with fluorescein. Click image to enlarge. |
DED Management
A healthy ocular surface is critical in supporting healthy, comfortable contact lens wear. Contact lenses divide the tear film into a pre- and post-lens tear film. This affects the biochemical properties of the tear film and results in a thinner lipid layer and increased tear evaporation.4 As such, you must proactively treat any patient with dry eye signs or symptoms prior to initiating a contact lens fit. Simple interventions such as lid hygiene and warm compresses may improve contact lens comfort and prevent future contact lens dropout.
According to the DEWS II guidelines, a stepwise approach is appropriate for dry eye management. First-level interventions include patient education, lid hygiene and artificial tears. For patients who remain symptomatic, step two recommendations include supplementing with overnight ointments, moisture chamber goggles, punctal plugs or in-office procedures like thermal compressive therapy such as LipiFlow (Johnson & Johnson Vision). Prescription medications that calm inflammation, including immunomodulators (e.g., cyclosporine, lifitegrast), steroids or oral tetracyclines, are also indicated in step two.
For patients who require additional intervention, step three considerations include autologous serum and therapeutic contact lenses. For severe cases who do not respond to more conservative treatments, consider amniotic membrane grafts, tarsorrhaphy or salivary gland transplantation.4
 |
|
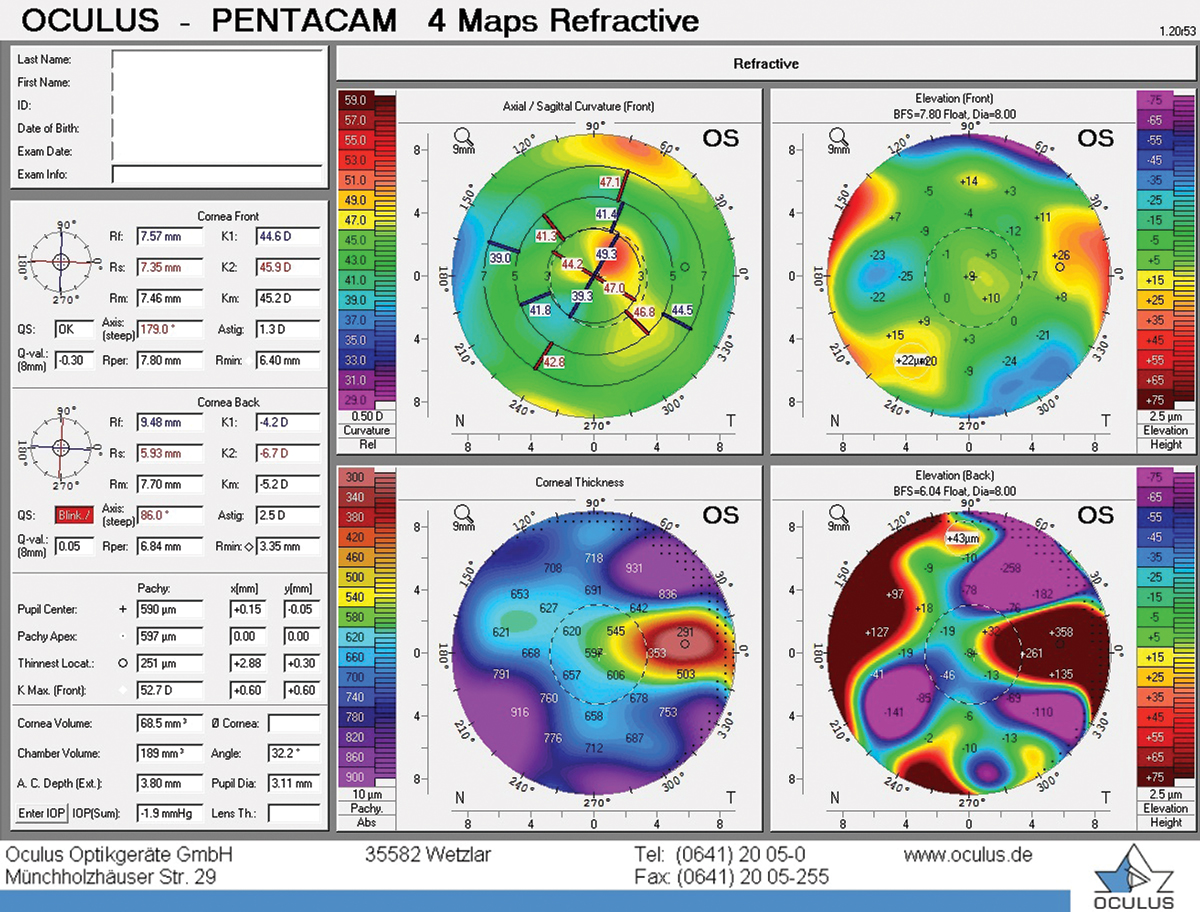
Irregular astigmatism on topography in a patient with OSD. Click image to enlarge. |
Contact Lens Fitting
After thoroughly assessing and determining the patient’s OSD severity, determine which lens option will benefit them.
Soft lenses. For patients with mild DED who are being managed appropriately, consider a soft contact lens fit. Ideally, select a daily disposable lens if available in the patient’s prescription. The daily disposable modality minimizes the risk of lens deposits and lens care systems affecting the ocular surface.
Daily disposables have also been found to cause less damage to the ocular surface and lower levels of inflammation.7 If the patient requires a reusable lens, recommend a hydrogen peroxide care system to minimize the risk of solution sensitivity.
As far as material selection, there have been several studies that refit hydrogel wearers into silicone hydrogel (SiHy) lenses and found less dryness, improved hyperemia and better comfort.8 Conversely, higher levels of inflammatory markers were found in the tears of SiHy wearers. This is due to the higher modulus and lower wettability of SiHy lenses.7 Many new SiHy materials have been created to combat this issue by promoting wettability and lowering the modulus while still maintaining high oxygen permeability.
 |
|
A scleral lens demonstrating a 1:1 ratio of lens thickness (dark band) to tear layer (green band). Click image to enlarge. |
It is easy to get overwhelmed with all the contact lens options we have nowadays. A few notable SiHy advancements include Alcon’s Dailies Total1 and Precision1 lenses, which feature a gradient design where water content increases towards the outside of the lens to promote lens wettability and patient comfort, and Bausch + Lomb’s Infuse lens, which also promotes wettability and reduces discomfort. Coopervision’s MyDay and Biofinity lenses also provide moisture and breathability. Johnson & Johnson’s 1-Day Oasys with Hydraluxe features a wetting agent that mimics tears to improve comfort. All of these advancements allow for increased wear time and improved end-of-day comfort.
During every follow-up visit, verify your patient’s lens care system and replacement schedule. Recently, one of my patients complained of severe dryness in his multifocal toric soft lenses. After further questioning, it turned out he had recently began using his partner’s gas permeable (GP) lens solution to clean and store his soft lenses. After reinforcing proper hygiene with an approved soft lens multipurpose solution, his symptoms greatly improved.
GP lenses. In moderate-to-severe cases of OSD, consider a specialty contact lens fit. GP lenses are an excellent choice for patients with high prescriptions or astigmatism with their sharp optics and high oxygen transmissibility. Orthokeratology, or corneal reshaping, is another GP option to consider for certain patients. Since these lenses are worn overnight, the patient does not need to be concerned with lens dryness during the day. The patient will also have easier access to the ocular surface for drop instillation.
Scleral lenses are an excellent treatment option for patients with moderate-to-severe cases of OSD who have failed with more conservative treatments. Studies have demonstrated the safety and efficacy of scleral lenses in improving ocular comfort, protecting the ocular surface and resolving epitheliopathy in patients with many types of OSD, including neurotrophic keratopathy, exposure keratopathy and limbal stem cell deficiency.9 Scleral lenses are large-diameter GPs that vault the cornea and provide excellent vision with their GP optics and stability.
Sclerals protect the cornea from the environment and bathe it with fluid. Patients with OSD may benefit from filling their sclerals with preservative-free artificial tears or a preservative-free saline mixed with several drops of preservative-free tears.
 |
|
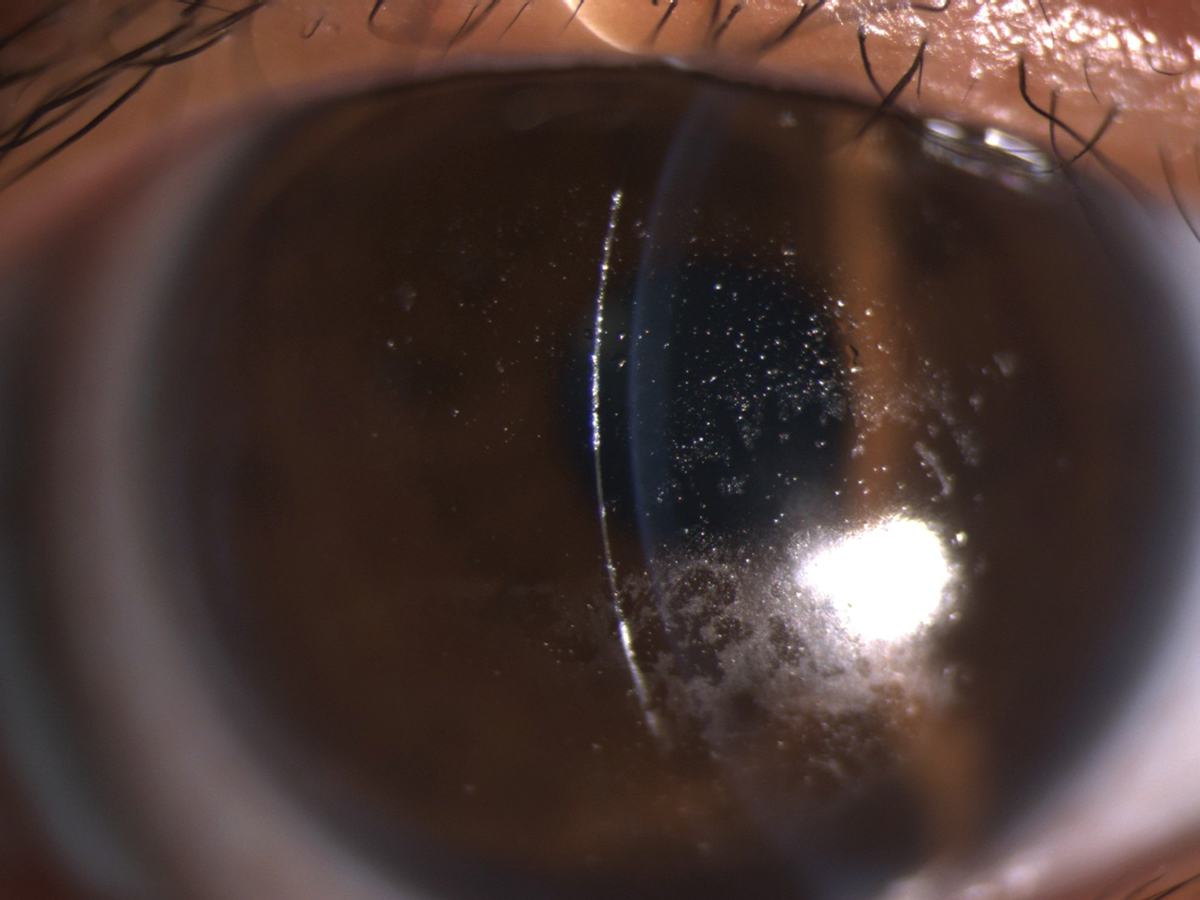
A Hydra-PEG coating would benefit this patient with heavy deposits on a scleral lens. Click image to enlarge. |
Fitting philosophies for scleral lenses vary, but remember that these are thicker lenses with a substantial tear layer. When fitting sclerals, use a highly oxygen permeable material. Lens vault is critical; excessive clearance will decrease oxygen to the cornea, but minimal clearance may cause the lens to settle and touch the cornea.
Similarly, lens thickness is crucial, as excessive amounts can decrease oxygen delivery, but minimal thickness may cause flexure and induce astigmatism.10 See your scleral patients back for follow-up after they have worn their lenses for several hours to determine the amount of clearance that remains after lens settling.
Many patients with OSD have fogging and wetting issues with their contact lenses. For these patients, be sure to confirm their lens replacement schedule, cleaning regimen, current dry eye regimen and lens wear time. Consider additional intervention with an extra-strength treatment like Progent (Menicon) or a special lens coating like Tangible Hydra-PEG (Tangible Science). The Hydra-PEG coating can be added to certain GP materials to minimize lens deposits and fogging while improving wettability and lubricity. Take caution to not use any abrasive lens cleaner that will strip this coating.
When establishing a follow-up schedule for your patients, consider the severity of their OSD and the type of lens they are wearing. For new lens wearers and patients fit in specialty lenses, monitor them, at minimum, every six months to re-evaluate their corneas and symptomatology. Monitor patients with moderate-to-severe OSD every one-to-three months after finalizing their contact lens fit. For milder cases, follow-up every six months may be sufficient.
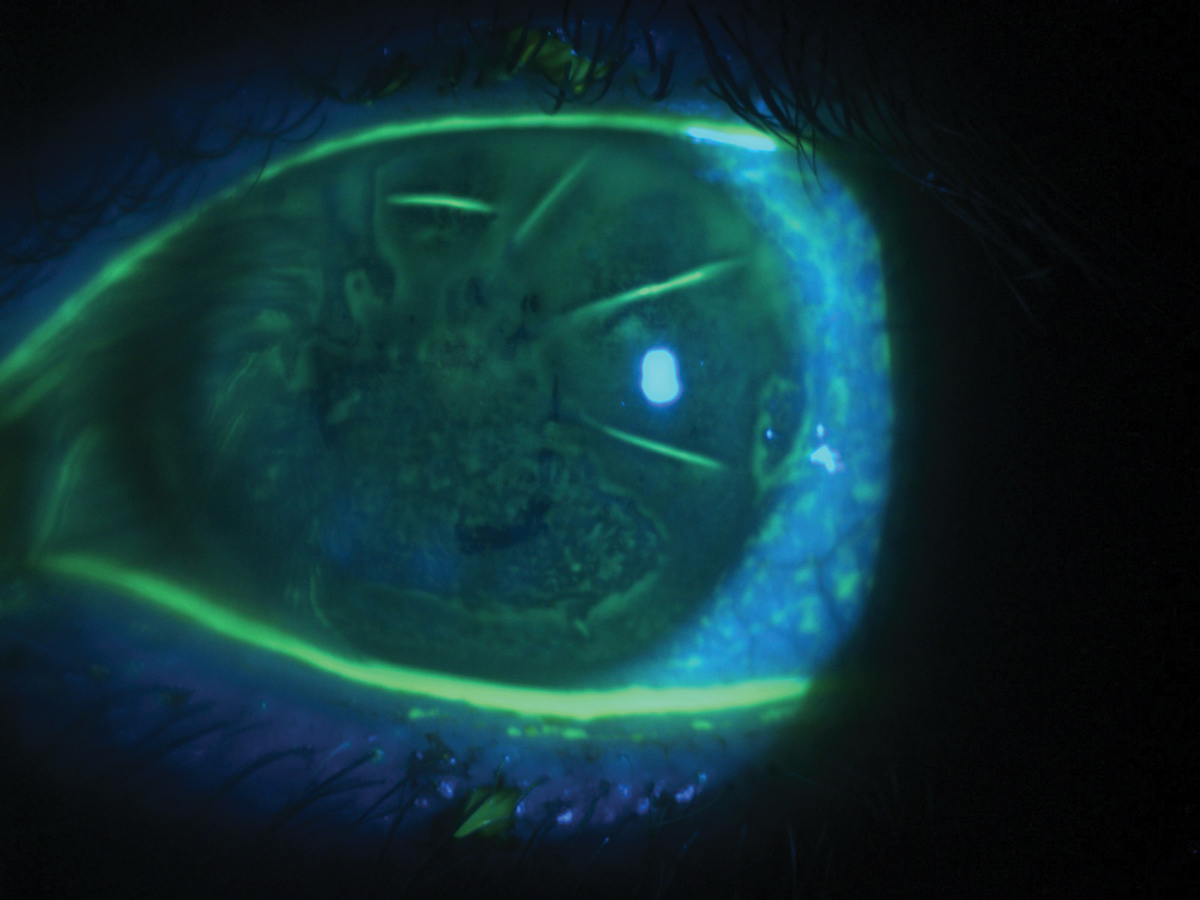 |
|
Diffuse corneal staining with fluorescein in a patient with severe dry eye and a history of radial keratotomy. Click image to enlarge. |
Case Study
A 42-year-old Hispanic male with a history of radial keratotomy (RK) and dry eye in both eyes was referred for a contact lens evaluation. His referral indicated a history of trialing soft and hybrid contact lenses with inadequate vision and comfort. His dry eye was managed with Xiidra (lifitegrast 5%, Shire) twice daily and preservative-free artificial tears four times daily in both eyes. Best-corrected spectacle vision was 20/40 in the right and left eyes. Externals were unremarkable. Slit lamp exam was remarkable for mild scurf, several capped meibomian glands, scattered RK scars and diffuse corneal staining with fluorescein in both eyes.
A scleral lens fit was initiated. In scleral lenses, the patient achieved 20/25 vision in each eye. The patient was urged to continue his current regimen of Xiidra and preservative-free tears as well as initiate warm compresses, lid hygiene and nightly ointment use. He has now been wearing scleral lenses successfully for two years. His corneal findings and symptoms have improved over this time.
Key Clinical Takeaways
Diagnosing and managing DED can be complicated, but managing the ocular surface upfront pays off in the long run and leads to more successful, satisfied contact lens patients. A careful assessment of the ocular surface could lead to increased success and reduced dropout rates.
Dr. Frantzis is an assistant clinical professor at SUNY College of Optometry, where she supervises interns in the Contact Lens and Myopia Control clinics. She also engages in clinical research with an emphasis on contact lens and myopia control studies. She has no financial interests to disclose.
1.Pucker AD, Jones-Jordan LA, Marx S, et al. Clinical factors associated with contact lens dropout. Cont Lens Anterior Eye. 2019;42(3):318-24. 2. Nichols KK, Nichols JJ, Mitchell FL. The lack of association between signs and symptoms in patients with dry eye disease. Cornea. 2004;23(8):762-70. 3. Finis D, Pischel N, König C, et al. Comparison of the OSDI and SPEED questionnaires for the evaluation of dry eye disease in clinical routine. Ophthalmologe. 2014;111(11):1060-6. 4. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II report. Ocul Surf. 2017;15(3):276-283. 5. Arita R, Fukuoka S, Morishige N. Meibomian gland dysfunction and contact lens discomfort. Eye Contact Lens. 2017; 43(1):17-22. 6. Sullivan BD, Crews LA, Sönmez B, et al. Clinical utility of objective tests for dry eye disease: variability over time and implications for clinical trials and disease management. Cornea. 2012:31(9):1000-8. 7. Muhafiz E, Bayhan HA, Şahin S, et al. Evaluation of the ocular surface in different contact lens replacement schedules. Cornea. 2019;38(5):587-94. 8. Chalmers R. Overview of factors that affect comfort with modern soft contact lenses. Cont Lens Anterior Eye. 2014;37(2):65-76. 9. Schornack MM, Pyle J, Patel SV. Scleral lenses in the management of ocular surface disease. Ophthalmology. 2014;121(7):1398-405. 10. Michaud L, van der Worp E, Brazeau D, et. al. Predicting estimates of oxygen transmissibility for scleral lenses. Contact Lens Anterior Eye. 2012;35(6):266-71. |

