
A 39-year-old white male, H.L., presented to clinic with a known history of glaucoma. His chief complaint was intermittent, shooting pain in both eyes. These episodes only lasted one or two seconds and occurred approximately once every two weeks.
H.L.s glaucoma diagnosis was made two months prior by a previous eye-care provider, an ophthalmologist, who had prescribed him Betimol (timolol ophthalmic solution, Vistakon Pharmaceuticals) b.i.d. O.D. The patient reported that he had run out of Betimol nearly one month ago and now wished to obtain his prescription through our clinic.
Additionally, H.L., a truck driver, reported a positive family history of glaucoma (affecting his father and grandfather), and that he smoked tobacco. His medical history was unremarkable.
Diagnostic Data
Upon examination, H.L. demonstrated a best-corrected visual acuity of 20/20 O.U., with a refractive error of -0.25 -0.50 x 105 O.D. and -0.25 -0.75 x 085 O.S. His pupils were equal, round and reactive to light with no relative afferent pupillary defect.
Anterior segment examination revealed 2+ endothelial pigment deposition O.U., consistent with the presentation of Krukenberg spindles. There were no signs of iris transillumination defects O.U. Intraocular pressure (IOP) was initially measured at 27mm Hg O.D. and 28mm Hg O.S. Four-mirror gonioscopy revealed open angles O.U. with 3+ iris processes and 4+ trabecular meshwork pigmentation O.U. The dilated fundus exam revealed a cup-to-disc ratio of 0.75H x 0.65V O.D. with inferior and nasal neuroretinal rim (NRR) thinning and 0.60H x 0.60V O.S. with no NRR thinning.
The lens, vitreous, posterior pole and peripheral retina were unremarkable O.U. Central corneal thickness measured 485-average O.D. and 478-average O.S. Repeatable threshold visual fields confirmed a superonasal step O.D. (see figures 1 and 2) and paracentral defects O.S.
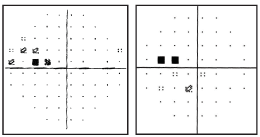
1,2. The patients visual fields, taken four months apart, show repeatable superonasal defects O.D.
Diagnosis
The clinical findings were consistent with pigmentary dispersion glaucoma (PDG) O.U. I suspected that H.L.s chief complaint of intermittent sharp eye pain was secondary to episodic increased pigment liberation with subsequent IOP spikes.
Treatment and Follow-Up
The patient was initially started on timolol 0.5% b.i.d. O.U. We scheduled the patient for follow-up.
Secondary Diagnosis
At the five-week follow-up examination, H.L.s IOP was 18mm Hg O.U., approximately a 35% reduction. But, the dilated fundus exam now revealed an inferotemporal optic disc hemorrhage (ODH) with a corresponding retinal nerve fiber layer (RNFL) wedge defect O.D. (see figures 3, 4 and 5).
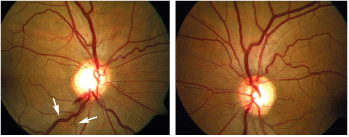
3,4. A fundus photo of the right eye shows an ODH at the inferotemporal rim with a corresponding RNFL wedge defect (left, see arrows). Right, a fundus photo of the left eye.
Further Follow-Up
Finding the ODH prompted us to set a new target IOP to mitigate any future glaucomatous damage. Combination therapy with Timolol and Travatan (travoprost, Alcon) q.h.s. O.U. was successful in reaching this target IOP, 12mm Hg, O.U. at the follow-up examination.
Now, H.L. is being monitored closely with visual fields, OCT and fundoscopy to determine if the current target IOP is sufficient to slow or halt further glaucomatous damage.
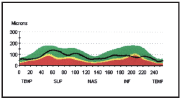
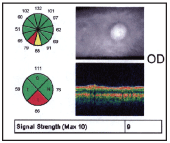
5. OCT of the right eye objectively confirmed RNFL thinning at the location of the ODH and RNFL wedge defect.
Discussion
Optic disc hemorrhages, also known as Drance hemorrhages, are often thought to be associated with normal-tension glaucoma (NTG). Although NTG patients are more likely to develop an ODH, it is not exclusive to this type of glaucoma.1-3 Patients with all forms of chronic open-angle glaucoma have been shown to have a higher prevalence of ODH compared with normal individuals, who rarely develop this condition.1-3
Chronic Open-Angle Glaucoma Subtypes Primary open-angle glaucoma Low tension High tension Secondary open-angle glaucoma Pigmentary Pseudoexfoliation
The prevalence of ODH in open-angle glaucoma has been reported to range from 5% to 58%. However, the Blue Mountains Eye Study, an Australian population-based study, has reported a prevalence estimate of 13.8%.1 Furthermore, studies have shown a higher prevalence of ODHs in primary open-angle glaucoma (POAG) over ocular hypertension, and an increased incidence of ODH after conversion from ocular hypertension (OHTN) to POAG (see Prevalence of ODH by Study," below).2,4
Prevalence of ODH by Study Blue Mountains Eye Study1 Kitazawa et al7 Normals 1.0% 0.4% Normal-tension glaucoma 25% 20.5% High-tension glaucoma 8% 4.2%
OHTN 1.5% 0.5%
These relationships suggest that the pathogenesis of an ODH is, to some extent, independent of IOP and that it is intrinsically connected with the process of glaucomatous optic neuropathy.
Clinically, an ODH may appear as a flame- or splinter-shaped hemorrhage that is oriented perpendicular to the optic disc margin. Optic disc hemorrhages are usually localized to the superotemporal and inferotemporal neuroretinal rim margin.5 They typically extend from within the optic nerve head to the adjacent peripapillary retina and may be associated with localized RNFL defects and neuroretinal rim notching.6 Resolution of an ODH can take from two to 35 weeks, with an average of 11 weeks.3,7 ODH recurrence rates vary greatly, ranging from 12% to 73%.8,9 This discrepancy in recurrence rates is thought to be due to differences in study parameters.
Pathogenesis of an ODH
The association between open-angle glaucoma and ODH is thought to be related to an underlying vascular anomaly. Proposed mechanisms of ODH, while still controversial, include ischemic microinfarction localized to the optic disc and mechanical shearing of small blood vessels secondary to morphological changes at the lamina cribrosa, as well as primary vascular dysregulation.2,10
Recently, a new hypothesis has postulated that primary vascular dysregulation (PVD) may be the underlying pathogenic mechanism.10 PVD is a multi-organ disease that mainly affects the microcirculation. It is characterized by cold hands, low blood pressure, orthostatic problems, tinnitus, disturbed sleep-onset, migraine, altered drug sensitivity and decreased thirst secondary to autonomic vessel dysfunction and endothelial cell dysfunction.11 While the etiology of PVD is unclear, these patients have been shown to have elevated serum levels of plasma peptides endothelin-1 (ET-1) and matrix metalloproteinases-9 (MMP-9). In this scenario, concurrent elevation of ET-1 and MMP-9 in sufficient quantity may bioactively compromise the peripapillary retinal-blood barrier, resulting in hemorrhage at the optic disc border.10
Aside from its role in ODH formation, PVD has been linked to open-angle glaucoma.10 Further, PVD is more common in NTG than in high-tension glaucoma (HTG), which parallels the prevalence trends seen in patients with an ODH.10 Such correlations further support the role of PVD in ODH formation. Elucidation of ODH pathogenesis may provide important insight to future open-angle glaucoma treatment and better our understanding of ODH as it relates to the glaucoma process.
Prognostic Signicance of an ODH
The most important implication of an ODH is its prognostic value for predicting progression of open-angle glaucoma. The presence of an ODH in patients with open-angle glaucoma usually signifies recent or imminent progression of visual field loss, subsequent RNFL damage, and notching of the neuroretinal rim.6,12 The latency of observed visual field loss and degree of visual field progression after an ODH varies by study, but most agree that the outcome is negative.9,12-14
One study showed that NTG patients who experience an ODH have a 20-fold increased risk of visual field loss progression.13 Another study showed that an ODH is equally ominous in NTG and POAG, in which approximately 80% of patients with an ODH showed VF deterioration vs. only 30% of patients who did not manifest an ODH.12
Although ODHs commonly present unilaterally, the unaffected eye is at equal risk for field loss progression in POAG, with a lower risk in NTG.12 Therefore, regardless of the type of open-angle glaucoma, the presence of an ODH is a warning sign for progression of the glaucomatous process that necessitates an aggressive management response. This is especially true for recurrent ODHs, which have even greater consequences for glaucomatous optic nerve damage, and may result in more profound visual field progression than in eyes with isolated ODHs.9,13
The association between pigmentary glaucoma and ODHs has been rarely reported.14 Typically, the glaucomatous process in pigmentary dispersion is thought to be a direct result of elevated IOP secondary to excessive pigment deposition into the trabecular meshwork. The presence of an ODH in our patient, however, may point to more than just an IOP-dependent glaucomatous process. Intuitively, one might think that the elevated IOP itself may have predisposed our patient to developing an ODH. But, the fact that ODH is more prevalent in NTG than POAG or OHTN would dispute this assertion. Conversely, one might think that our patient developed the ODH secondary to a significant drop in the IOP following initiation of timololbut this theory would be contradicted by research that shows a decreased incidence of ODH in patients with POAG following trabeculectomy.14 Or, it simply may be that our patient developed an ODH due to structural deterioration of the optic nerve with subsequent shearing of optic nerve blood vessels. Continued research on biomarkers and their role in ODH formation may clarify why ODH can be found in different mechanisms of glaucoma.7
The significance of an ODH in pigmentary glaucoma has not been well defined, but the mere presence of an ODH in any type of open-angle glaucoma serves as a warning sign for possible glaucoma progression. Furthermore, it is imperative that clinicians identify the presence of an ODH in glaucoma patients during routine examination. This is best achieved by performing undilated fundoscopy with each IOP check and by using fundus photography during dilated exams, as ODHs are often overlooked with dilated fundoscopy alone.4
What Does It All Mean?
An ODH finding in the presence of pigmentary glaucoma raises interesting questions about the etiology of both conditions, and further studies are needed to understand these enigmatic processes. An ODH portends progressive glaucomatous damage regardless of the type of open-angle glaucoma the patient exhibits.8 These patients should be monitored frequently and treated aggressively to minimize subsequent glaucoma damage associated with ODH.
Thankfully, the patients visual fields have stabilized, and his IOP is now under control.
Dr. Anthony currently practices at the
1. Healey P, Mitchell P, Smith W, Wang J. Optic disc hemorrhages in a population with and without signs of glaucoma. Ophthalmology 1998 Feb;105(2):216-23.
2. Jonas J, Xu L. Optic disk hemorrhages in glaucoma. Am J Ophthalmol 1994 Jul;118(1):1-8.
3. Soares A, Artes PH, Andreou P, et al. Factors associated with optic disc hemorrhages in glaucoma. Ophthalmology 2004 Sep;111(9):1653-7.
4. Budenz DL,
5. Jonas J, Martus P, Budde W, Hayler J. Morphologic predictive factors for development of optic disc hemorrhages in glaucoma. Invest Ophthalmol Vis Sci 2002 Sep;43(9):2956-61.
6. Sugiyama K, Uchida H, Tomita G, et al. Localized wedge-shaped defects of retinal nerve fiber layer and disc hemorrhage in glaucoma. Ophthalmology 1999 Sep;106(9):1762-7.
7. Kitazawa Y, Shirato S, Yamamoto T. Optic disc hemorrhage in low-tension glaucoma. Ophthalmology 1986 Jun;93(6):853-7.
8. Wilson MR, Creighton MS. Normal tension glaucoma. Acta Ophthalmologica Scand Suppl 2002;236:9-11.
9. Kim SH, Park KH. The relationship between recurrent optic disc hemorrhage and glaucoma progression. Ophthalmology 2006 Apr;113(4):598-602.
10. Grieshaber MC, Terhorst T, Flammer J. The pathogenesis of optic disc splinter haemorrhages: a new hypothesis. Acta Ophthalmol Scand 2006 Feb:84(1):62-8.
11. Greishaber M, Flammer J. Does the blood-brain barrier play a role in glaucoma? Surv Ophthalmol 2007 Nov;52:S115-S121.
12. Rasker MT, van den Enden A, Bakker D, Hoyng PF. Deterioration of visual fields in patients with glaucoma with and without optic disc hemorrhages. Arch Ophthalmol 1997 Oct;115(10):1257-62.
13. Ishida K, Yamamoto T, Sugiyama K, Kitazawa Y. Disk hemorrhage is a significantly negative prognostic factor in normal-tension glaucoma. Am J Ophthalmol 2000 Jun;129(6):707-14.
14. Miyake T, Sawada A, Yamamoto T, et al. Incidence of disc hemorrhages in open-angle glaucoma before and after trabeculectomy. J Glaucoma 2006 Apr;15(2):164-71.

