Today, optometrists play a crucial role in managing diabetes, a leading—and growing—instigator of vision loss. With management of this disease now firmly in optometry’s wheelhouse, the depth of research into its ocular impact has provided the ability to delineate its progression using various categories. In the case of diabetic retinopathy, these are divided, chiefly, into two: proliferative diabetic retinopathy and nonproliferative diabetic retinopathy. These categories are each further split by severity. It may seem like minutiae, but even minor distinctions can be valuable as they inform our treatment protocol and, ultimately, prevent significant visual impairment for our patients.
This article explains the care diabetes patients require and details the biological processes that indicate where to classify a patient with diabetic retinopathy, as well as what treatment should follow.
Risk Factors
Two particular aspects of diabetes can put patients at risk for developing diabetic retinopathy: duration and glycemic control.
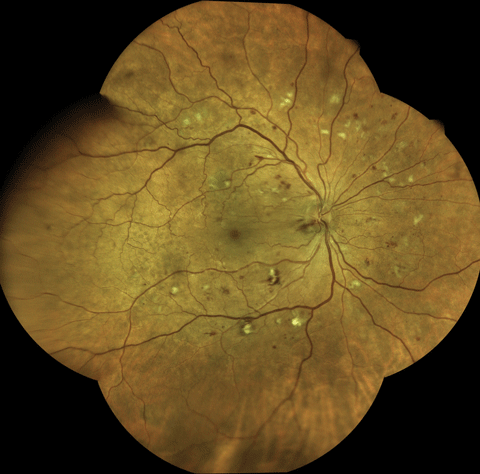 |
| Using an Eidon True Color Confocal Scanner, this image shows moderate to severe NPDR with multiple cotton-wool spot and dot/blot hemorrhages. Click image to enlarge. |
• Duration. Approximately 25% of Type 1 patients have some retinopathy after five years.7,8 These numbers increase to almost 60% after 10 years and greater than 80% after 15 years.7,8 In Type 2 patients older than age 30 with a known duration of diabetes of less than five years, 40% of patients taking insulin and 24% of those not taking insulin are found to have retinopathy. After 10 years, the numbers increase to 84% and 53%, respectively. Proliferative diabetic retinopathy is found in approximately 2% of type 2 patients who have diabetes for less than five years, and 25% who have had diabetes for 25 years or more.9
• Glycemic control. Multiple clinical studies, as well as epidemiologic studies, support this association. For example, the United Kingdom Prospective Diabetes Study revealed intensive blood sugar control in newly diagnosed patients with Type 2 diabetes had less microvascular complications, including retinopathy, compared with patients who received standard treatment.10,11 For every 1% decrease in HbA1c, there was a corresponding 35% risk reduction in retinopathy.10
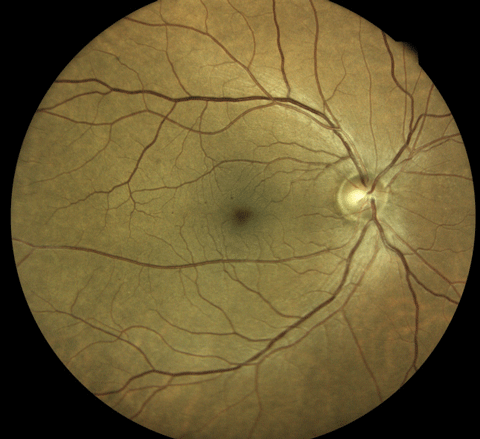
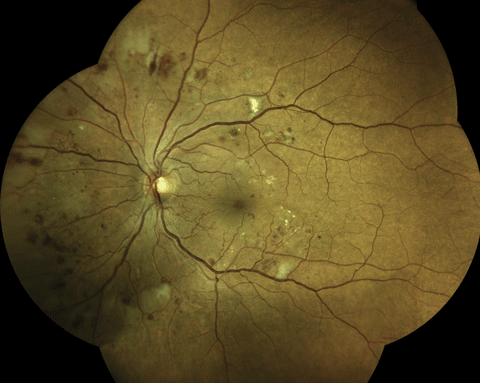 An Eidon True Color Confocal Scanner was used to image this PDR with an area of NVE at 10 o’clock. Below, this image shows a patient with mild nonproliferative diabetic retinopathy. Note the three dot hemorrhages superior to the macula. Click image above to enlarge. An Eidon True Color Confocal Scanner was used to image this PDR with an area of NVE at 10 o’clock. Below, this image shows a patient with mild nonproliferative diabetic retinopathy. Note the three dot hemorrhages superior to the macula. Click image above to enlarge. |
 |
Diabetes in AmericaAn estimated 25.6 million Americans age 20 or older have diabetes, with a third still undiagnosed.2 An additional 79 million people have prediabetes, and are at risk for developing diabetes.2,3 Researchers estimate the prevalence rate for diabetic retinopathy among adults with diabetes older than 40 years in the United States is 28.5%— 4 million Americans.2,4 Further, the rate of vision-threatening diabetic retinopathy in the United States is 4.4% or 0.7 million.5 If the overall rate of diabetes continues to rise at the current trajectory, the projected prevalence of individuals with any diabetic retinopathy by 2020 will increase to 6 million, with 1.34 million having sight- threatening disease.4,5 Type 1 diabetes is characterized by an absolute lack of endogenous insulin. These patients must be on exogenous insulin injections to survive. Type 1 accounts for only 5% of diabetes in the United States, and is generally diagnosed in children and young adults, although it can occur at any age.2 Type 2 diabetes is characterized by insulin resistance or the inability of the body to use the insulin it makes effec- tively. This accounts for 90% to 95% of all diabetes, and develops more frequent- ly in adults.2 However, due to childhood obesity and other factors, the prevalence of Type 2 diabetes is increasing in chil- dren and teens.6 Typical treatment starts with diet and exercise, then proceeds to oral medications. Some patients with Type 2 diabetes may take insulin as well, although typically do not need it for sur- vival, like patients with Type 1. Because of the higher proportion of patients with Type 2 diabetes, it accounts for the largest number of patients with visual loss from diabetic retinopathy, even though patients with Type 1 typically suffer from more frequent and more serious ocular complications. |
Once retinopathy is present, glycemic control becomes the more important factor in predicting the progression to advanced stages.12,13 In general, a HbA1c of 7% or less is recommend for most patients with diabetes.14 The management of hypertension, as well as lipids, has also been shown to reduce the progression of retinopathy, and delay the need for treatment.15-17
Patients with retinopathy should be counseled about these risk factors and encouraged to work with their physicians to obtain optimal control of their diabetes, as well as other associated medical issues.
Regular Check-ups
All patients with diabetes should have regular eye examinations. Patients with Type 1 diabetes without known retinopathy should have dilated retinal examinations beginning five years after their initial diagnosis, and annually thereafter.7,18
Type 2 patients should be examined shortly after their initial diagnosis, as often they have had the disease for several years before diagnosis.19 In fact, retinopathy is detected in 20% to 39% of patients upon initial diagnosis of Type 2 diabetes, and up to 3% of patients are found to have clinically significant macular edema (CSME) or high-risk retinopathy upon initial exam if their diabetes is diagnosed after age 30.7,9,20 Patients with no retinopathy should be counseled regarding the importance of routine examinations, even those with good vision and no symptoms. Approximately 5% to 10% of patients without retinopathy will develop it within one year.21,22 Those with retinopathy should be examined more frequently, depending upon their level and severity of involvement.
At the initial exam, as well as subsequent exams, a thorough history, including type of diabetes, duration of disease, glycemic control, current medications, current medical history (with particular attention to comorbid diseases such as hypertension, hyperlipidemia, obesity), and ocular history (including previous treatment for diabetic eye disease), should be obtained. Any retinal findings consistent with diabetic retinopathy in an undiagnosed patient warrants further investigation including lab tests, such as fasting blood sugar or glycosylated hemoglobin, and referral to primary care, as appropriate.
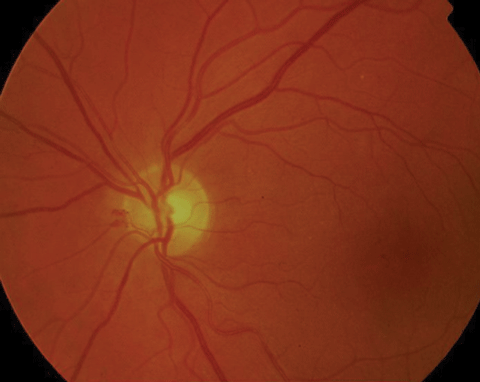 |
| Above, PDR with early NVD at 9 o’clock. Below, high-risk PDR with significant NVD greater than 1/3DD. Click images to enlarge. |
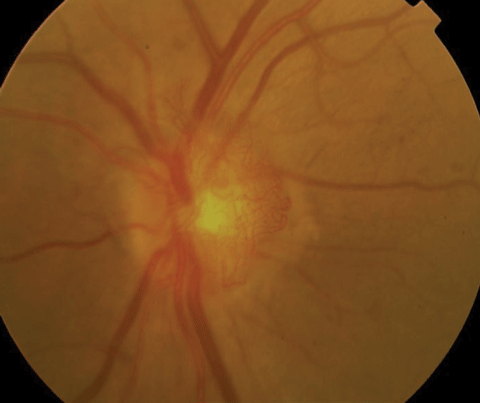 |
Diabetic retinopathy can worsen during pregnancy, either due to physiologic changes in pregnancy itself or changes in overall glycemic control.23-25 Therefore, patients with diabetes who plan to become pregnant should have a comprehensive eye exam prior to pregnancy, and be informed of the increased risk of retinopathy or its progression. A dilated retinal exam should be performed within the first trimester, with appropriate follow-up depending on presence and severity of retinopathy, if discovered. Conversely, women who develop gestational diabetes do not require an examination, as they do not appear to be at increased risk of retinopathy during their pregnancy.26
Physical Examination
When a diabetes patient presents to your office, perform a complete ophthalmic exam—including examination of the peripheral retina and vitreous through a dilated pupil. Pupil dilation is essential as research shows only 50% of eyes are correctly classified for the presence and severity of retinopathy through an undilated pupil.27
Ancillary testing methods—such as gonioscopy, optical coherence tomography (OCT) and fundus photos—should be performed when indicated, on a case-by-case basis. The presence or absence of any signs of retinopathy, such as hemorrhages, venous beading (VB), cotton-wool spots and retinal neovascularization, as well as exudates and thickening in the macula, should be documented.
Retinopathy Patients
Two major classifications of this disease exist: nonproliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy (PDR).
NPDR is characterized by retinal vascular abnormalities, such as microaneurysms, intraretinal hemorrhages, venous dilation, and cotton-wool spots. Intrarertinal microvascular abnormalities (IRMA) may also occur at this stage. NPDR is divided into mild, moderate, severe and very severe.
As the retinopathy progresses, gradual closure of the retinal vessels may occur, leading to decreased perfusion and retinal ischemia. Proliferative diabetic retinopathy can then follow, characterized by neovascularization, either of the optic disc (NVD) or elsewhere in the retina (NVE). These neovascular vessels are weak and fragile, and can rupture or bleed, leading to preretinal or vitreous hemorrhages, and subsequent vision loss. PDR can be further divided into PDR and high risk PDR.
• Mild NPDR. This classification is characterized by the presence of at least one retinal microaneurysm or hemorrhage. Microaneurysms are outpouchings of retinal capillary walls, caused by loss of pericytes of the cell wall—which leads to weakening. Hemorrhages result from leaking or ruptured microaneurysms deep within the retina, where the cells are compact and vertically oriented, leading to the characteristic pinpoint or dot/blot shape.
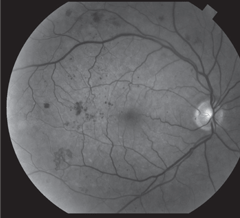 |
| Above, FA image demonstrates hyperfluorescence consistent with NVE. Below, this red-free photo shows PDR with NVE at 8 o’clock. Click images to enlarge. |
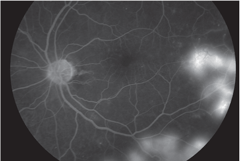 |
On clinical exam, a microaneurysm and hemorrhage appear quite similar and can truly only be differentiated by fluorescein angiography (FA), with microaneurysms appearing hyperfluorescent and hemorrhages appearing hypofluorescent. However, the difference between the two is clinically insignificant, as both point out the beginning stages of diabetic damage, and patients with mild NPDR should be reexamined in one year. Approximately 5% to 10% will increase to further stages of retinopathy over the course of a year.21,22 FA and laser are not indicated, nor are color photos necessary, but are often helpful in establishing a baseline to look for future progression, and can be useful in patient education.
• Moderate NPDR. This is characterized by increasing hemorrhages and microaneurysms as well as cotton wool spots, VB or IRMA to a mild degree. IRMAs represent either new vessel growth deep within the retina, or more likely, pre-existing vessels that serve to shunt blood through areas of nonperfusion. Patients with this level of retinopathy should be re-examined in six months, due to the increased disease progression, with approximately 16% of patients with moderate NPDR progressing to PDR within four years.21
As with mild NPDR, laser and FA are not indicated, but photos may be helpful to monitor for future progression.
• Severe NPDR. You can categorize this version of the condition by using the “4-2-1” rule—that is, one has severe NPDR if hemorrhages or microaneurysms, or both, appear in all four retinal quadrants; venous beading appears in two or more retinal quadrants; or prominent IRMAs are present in at least one retinal quadrant.
• Very severe NPDR. The most severe classification is reached when any two or more of these criteria are met. These patients should be followed extremely closely, within two to four months, due to the high risk of progression to proliferative disease. Approximately half of the patients with severe NPDR will progress to PDR within a year—and, of those, 15% will exhibit high-risk characteristics.28 For patients with very severe NPDR, the risk for PDR is approximately 75% within a year, with 45% becoming high risk.28
Due to the high rate of PDR progression in these patients, it is also reasonable to consider a consult to a retinal specialist at this level of retinopathy to consider treatment. This should especially be considered if there are extenuating circumstances, such that the patient cannot or will not be followed closely, or there are other considerations, such as pregnancy or impending cataract surgery.
Analysis shows that the risk of severe vision loss or need for vitrectomy was reduced by 50% in patients with Type 2 diabetes who received panretinal photocoagulation (PRP) treatment at this stage vs. waiting until high-risk PDR developed.29 Currently, the role of anti-VEGF treatment in the management of severe and very severe NPDR is being studied.
FA is often helpful at this stage to determine the presence of non-perfusion, peripheral ischemia or any clinically undetected areas of neovascularization, making a referral to a retinal specialist and treatment consideration even more important.
PDR
The hallmark of PDR is neovascularization, either on or within one disc diameter (DD) of the optic disc (NVD) or elsewhere in the retina (NVE); a preretinal hemorrhage (PRH); or vitreous hemorrhage (VH).
High-risk PDR is characterized by NVD greater than one-fourth to one-third disc area in size; any NVD with a vitreous or preretinal hemorrhage; or NVE greater than one-half a disc area in size with a PRH or VH. Any patient with PDR or high-risk PDR should be referred promptly to a retinal specialist for treatment, preferably within one week for PDR and one to two days for high-risk PDR. Without appropriate treatment, approximately 50% of eyes with PDR are blind within five years.28
The risk of severe vision loss in patients with high-risk PDR has been shown to be reduced substantially when treated with PRP.28,29 Therefore, most patients with PDR should receive PRP in hopes of causing regression of the retinal neovascularization. Newer studies have demonstrated that anti-VEGF agents such as Lucentis (ranibizumab, Genentech) may be a superior alternative to PRP laser, alone or when done in conjunction with conventional laser.30 For patients who fail to have vessel regression with laser or anti-VEGF treatment, a vitrectomy may be necessary.
Macular Edema
Another issue that must be addressed in diabetes patients is diabetic macular edema (DME), or accumulation of intraretinal fluid in the macula. While it is important to assess for diabetic retinopathy, it is equally as important to look for any evidence of DME. In fact, more patients with Type 2 diabetes suffer moderate vision loss from DME than frank retinopathy. Therefore, a careful assessment of the macula for exudates or signs of thickening is crucial in all patients with diabetes, even those with seemingly good visual acuity, as macular thickening only shows moderate correlation with visual acuity.31
Diabetic Retinopathy Level and Management | |||
| Level of Retinopathy | Retinal Findings | Additional Testing | Follow-Up/Referral |
| No Retinopathy | None | None | Twelve Months |
| Mild NPDR | ≥1 MA/Heme | Fundus photo‡ | Twelve Months |
| Moderate NPDR | MAs/Hemes, CWS, VB, mild IRMA -at level < Severe NPDR | Fundus photo‡ | Six Months |
| Severe NPDR | 4-2-1 Rule: MAs/hemes in 4 quadrants -or- VB in ≥ 2 quadrants -or- Prominent IRMA in ≥ 1 quadrant | Fundus photo FA‡ | Two months to four months retina referral‡ |
| Very Severe NPDR | Two or more of the above “4-2- 1” Criteria | ||
| PDR | Any NVD, NVE, PRH, VH | Fundus photo FA | Retina referral within one week |
| High Risk PDR | NVD >1/4 to 1/3DD -or- Any NVD with VH or PRH -or- NVE > 1/2DD with VH or PRH | Fundus photo FA | Retina referral within one day to two days |
| With CSME Non CI-Macular Edema CI-Macular Edema | Thickening within 500 μm (1/3DD) of the macular center -or- | Fundus photo FA macular OCT | Non center- involved macular edema: Two months to four months Center-involved macular edema: retina referral within one week to two weeks |
| ‡=Recommended NPDR=nonproliferative diabetic retinopathy; PDR=proliferative diabetic retinopathy; MA=microaneurysms; Hemes=hemorrhages; CWS=cotton wool spots; VB=venous beading; IRMA=intra-retinal microvascular abnormalities; NVD=neovascularization of the disc; NVE=neovascularization elsewhere; VH=vitreous hemorrhage; PRH=preretinal hem- orrhage; CSME=clinically significant macular edema; CI=centrally involving; DD=disc diameter | |||
Lastly, remember that diabetic macular edema can occur at any level of retinopathy, from mild NPDR to proliferative disease.
Traditionally, diabetic macular edema was classified as CSME if any of the following criteria were met:
1. Thickening of the retina at or within 500µm (1/3DD) of the center of the macula;
2. Hard exudates at or within 500µm (1/3DD) of the center of the macula with thickening of the adjacent retina;
3. A zone or zones of retinal thickening greater than 1 disc area in size, any portion of which is within 1DD of the center of the macula.32
With the advancement of OCT, some clinicians now prefer to subdivide macular edema according to the involvement at the center of the macula—opting for the term “center-involving diabetic macula edema” when it is centrally located, and “non-center involving edema” when the center of the macula is spared. In general, the risk for vision loss and need for treatment is greater with central involvement.
The diagnosis of DME can be difficult even for experienced clinicians. It is best viewed through a dilated pupil using slit lamp biomicroscopy and appropriate lenses, or stereo photos. OCT is also particularly helpful for detecting subtle edema, as well as in following the edema after treatment for resolution. However, studies indicate that routine OCT imaging is not indicated in patients with minimal diabetic retinopathy when no retinal thickening is suspected on clinical exam.33
Any patient suspected of having center-involving DME should be referred to a retinal specialist for consideration of treatment within one to two weeks. FA prior to treatment is helpful to identify those lesions amenable to treatment. Also, it is useful to detect areas of capillary dropout around the macula and enlargement of the foveal avascular zone, which may be useful in deciding the most appropriate treatment.32
Patients with non-center involving DME should be followed closely, every two to four months, with repeat OCTs as needed, with a prompt referral when the central macula begins to become involved. Also, a referral to the patients’ primary care physician to optimize glycemic control as well as other associated issues is warranted.
Traditional treatment of CSME has been laser surgery. Early studies showed that using focal laser for patients with center-involving CSME would decrease the loss of by 50% vs. observation alone.32 Further, it was shown that patients with center-involving CSME had a ten-fold greater risk of moderate vision loss at one year compared with those patients without center involvement.
However, data from several more recent, well-designed studies reveal that intravitreal anti-VEGF agents may provide more effective treatment for center-involving CSME than laser therapy alone.34,35,36 Therefore, referral to a retinal specialist well-versed in the latest studies and protocols for treating CSME is advised.
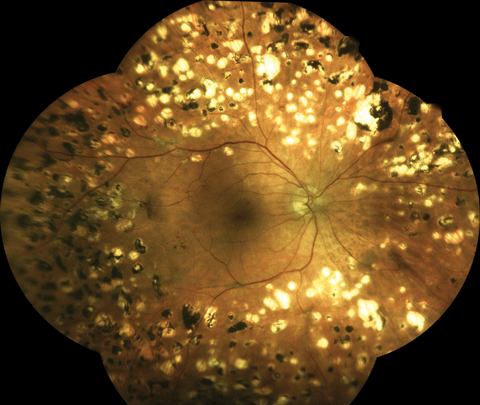 |
| Taken with an an Eidon True Color Confocal Scanner, this image shows a patient following extensive panretinal photocoagulation for proliferative diabetic retinopathy. Click image to enlarge. |
Monitoring
Since the majority of patients with diabetes will develop some retinal involvement during the course of the disease, the importance of careful examination with a dilated retinal exam and appropriate follow-up or treatment when indicated cannot be overstated. Referral to a retinal specialist at the onset of sight threating, treatable retinopathy is crucial. Studies indicate that early detection and prompt treatment of diabetic retinopathy is approximately 90% successful in preventing severe vision loss (visual acuity less than 5/200). However, according to several studies, it appears that only about 50% of patients with diabetes are receiving timely dilated eye examinations.37
Patients with diabetes should be counseled regarding the visual side effects of their disease, and be encouraged to report any symptoms associated with progressive disease, such as decreased vision or floaters. They should be educated that retinopathy may occur even with normal vision, in the absence of any symptomatology, highlighting the need to adhere to your recommended recall and routine examinations. Patients should be encouraged to work with their other physicians to obtain good glycemic control as well as control of other associated diseases, such as hypertension, to reduce the risk of retinopathy or its progression. Lastly, those patients who suffer vision loss despite our best efforts should be referred to a provider specializing in low vision.
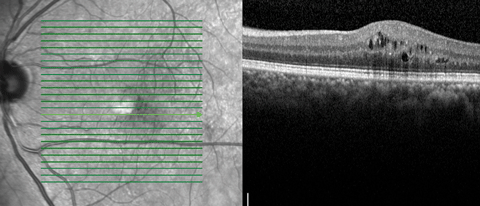 |
| OCT image demonstrating mild CSME temporal to the macula, left eye. |
Hopefully, with increased vigilance and better communication, we can work with our patients with diabetes to prevent the devastating effects of this disease and help our patients lead healthy and productive lives.
Dr. Ferrucci is chief of optometry and residency director at the Sepulveda VA Ambulatory Care Center in North Hills, Calif. He is also a professor at Southern California College of Optometry at Marshall B. Ketchum University and a consultant to Eidon.
Dr. Yeh is a staff optometrist at the VA Sepulveda Ambulatory Care Center and an adjunct faculty member at Southern California College of Optometry at Marshall B. Ketchum University.
|
1. Klein BE. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol. 2007;14:179-83. 2. Centers for Disease Control and prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the US, 2011. 3. Cowie C, Rust K, Byrd-Holt D, et al. Prevalence of diabetes and impaired fasting glucose in adults in the US population: National Health and Nutrition Examination Survey. 1999-2002. Diabetes Care. 2006;29:1263-8. 4. Kempen J, O’Colmain B, Leske M, et al. The prevalence of diabetic retinopathy among adults in the United States. Arch Ophthalmol. 2004;122:552-63. 5. Zhang X, Saddine J, Chou C, et al. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA 2010; 304:649-656. 6. Fagot-Campagna A, Pettitt D, Engelgau MM, et al. Type 2 diabetes among North American Children and adolescents: an epidemiologic review and a public health perspective. J Pediatr. 2000;136;664-72. 7. Klein R, Klein B, Moss S, et al. The Wisconsin epidemiologic study of diabetic retinopathy II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol. 1984;102:520-6. 8. Varma R, Torres M, Pena F, et al. Prevalence of diabetic retinopathy in adult Latinos: The Los Angeles Latino Eye Study. Ophthalmology 2004;111:1298-306. 9. Klein R, Klein B, Moss S, et al. The Wisconsin epidemiologic study of diabetic retinopathy III. Prevalence and risk of diabetic retinopathy when age at diagnosis is more than 30 years. Arch Ophthalmol. 1984;102:527-32. 10. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulfonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes. Lancet. 1998;352:837-53. 11. UK Prospective Diabetes Study VIII. Study design, progress, and performance. Diabetologia. 1991;34:877-90. 12. Davis M, Fisher M, Gangnon R, et al. Risk factors for high-risk proliferative diabetic retinopathy and severe vision loss: Early Treatment Diabetic Retinopathy Study report number 18. Invest Ophthalmol Vis Sci. 1998;39:233-52. 13. Kilpatrick ES, Rigby AS, Atkin SL, Frier BM. Does severe hypoglycemia influence microvascular complications in type 1 diabetes? An analysis of the Diabetes Control and Complications Trial database. Diabet Med. 2012;29:1195-8. 14. American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care. 2013;336 Suppl1:s11-66. 15. UK Prospective Diabetes Study Group. Tight Blood Pressure control and risk of macrovascular and microvascular complications I type 2 diabetes: UKPDS 38. BMJ 1998;317:703-13. 16. Snow V, Weiss K, Mottur-Pilson C. The evidence for tight blood pressure control in the management of type 2 diabetes mellitus. Ann Intern Med 2003;138:587-92. 17. Klein R, Sharrett A, Klein B, et al. The association of atherosclerosis, vascular risk factors, and retinopathy in adults with diabetes: the atherosclerosis risk in communities study. Ophthalmology. 2002;109:1225-34. 18. Lueder G, Silverstein J. American Academy of Pediatrics section on Ophthalmology and Endocrinology in the pediatric patient with type 1 diabetes. Pediatrics 2005;116:270-273. 19. Klein R, Klein B, Moss S. Epidemiology of proliferative diabetic retinopathy. Diabetes Care. 1992;15:1875-91. 20. Diabetes Prevention Program Research Group. The prevalence of retinopathy in impaired glucose tolerance and recent-onset diabetes in the diabetes prevention program. Diabet Med. 2007;24:137-44. 21. Klein R, Klein B, Moss S, et al. The Wisconsin epidemiologic study of diabetic retinopathy IX. Four-year incidence and progression of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol. 1989;107:237-43. 22. Klein R, Klein B, Moss S, et al. The Wisconsin epidemiologic study of diabetic retinopathy X. Four-year incidence and progression of diabetic retinopathy when age at diagnosis is 30 years or more. Arch Ophthalmol. 1989;107:244-9. 23. Klein B, Moss S, Klein R, et al. Effect of pregnancy on progression of diabetic retinopathy. Diabetes Care. 1990;13:34-40. 24. Chew EY, Mills JL, Metzger BE, et al. Metabolic control and progression of retinopathy. The diabetes in early pregnancy study. National Institute of Child Health and Human Development Diabetes in Early Pregnancy Study. Diabetes Care. 1995; 18:631-7. 25. Diabetes control and complication trial research group. Effect of pregnancy on microvascular complications in the diabetes control and complications trial. Diabetes Care. 2000;23;1084-91. 26. Gunderson E, Lewis E, Tsai A, et al. A 20-year prospective study of childbearing and incidence of diabetes in young women, controlling for glycemia before conception: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Diabetes. 2007;56:2990-6. 27. Klein R, Klein B, Neider M, et al. Diabetic retinopathy as detected using ophthalmology, a nonmydriatic camera and a standard fundus camera. Ophthalmology. 1985;92:485-91. 28. Early Treatment Diabetic Retinopathy Study Research Group. Early photocoagulation for diabetic retinopathy: ETDRS report number 9. Ophthalmology. 1991;98:766-85. 29. Ferris F. Early photocoagulation in patients with either type I or type II diabetes. Trans Am Ophthalmol Soc. 1996;94:505-37. 30. Writing Committee for the Diabetic Retinopathy Clinical Research Network. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA. 2015;314:2137-46. 31. Nunes S, Pereira I, Santos A. Central retinal thickness measured with HD-OCT shows a weak correlation with visual acuity in patients with CSME. Br j Ophthalmol 2010;94:1201-4. 32. Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. ETDRS report No. 4. Int Ophthalmol Clin. 1987;27:265-72. 33. Browning DJ, Fraser CM, Clark S. The relationship of macular thickness to clinically graded diabetic retinopathy severity in eyes without clinically detected diabetic macular edema. Ophthalmology. 2008;115:533-9. 34. Ho AC, Scott IU, Kim SJ, et al. Anti-vascular endothelial growth factor pharmacotherapy for diabetic macula edema: a report by the American Academy of Ophthalmology. Ophthalmology. 2012;119:2179-88. 35. Mitchell P, Bandello F, Schmidt-Erfurth U, et al. RESTORE Study Group: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118:615-25. 36. Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results form 2 phase III randomized clinical trials: RISE and RIDE. Ophthalmology. 2012;119:789-801. 37. Paz SH, Varma R, Klein R, et al. Los Angeles Latino Eye Study Group. Noncompliance with vision care guidelines in Latinos with type 2 diabetes mellitus: the Los Angeles Latino Eye Study. Ophthalmology. 2006;113:1372-7. |

