Retina Takes the StageExperts offer pearls for detecting and managing numerous posterior segment conditions in the June 2024 issue of Review of Optometry, our 15th annual Retina Report. Check out the other articles featured in this issue:
|
In recent decades, rates of diabetes have climbed to epidemic levels. The most current report from the CDC released in 2021 states that 38.4 million individuals in the US have diabetes, accounting for nearly 12% of the total population.1 Diabetic retinopathy (DR) is a common complication of diabetes mellitus (DM), affecting approximately 30% of adults with diabetes, and is the leading cause of blindness in working-aged Americans.2 As primary eyecare providers, the role of optometric physicians is more important than ever to prevent devastating vision loss amongst our growing diabetic population. This article will review the most recent literature—as well as provide anecdotal insights from our hands-on experience with thousands of cases—on causes, presentations and diagnostic techniques in diabetic eye disease.
Pathogenesis and Clinical Findings
Inner retinal microvascular changes due to prolonged hyperglycemia can be visualized in the retina as microaneurysms, hemorrhaging, leakage and exudation and nonperfusion. These findings fall into the category of nonproliferative changes. More advanced features of nonproliferative disease include venous beading, intraretinal microvascular abnormalities (IRMA) that represent dilated telangiectatic capillaries still confined intraretinally and vascular sheathing. As DR continues to advance, mounting retinal ischemia prompts vascular endothelial growth factor (VEGF) release, fueling preretinal neovascular growth that characterizes the proliferative retinopathy stage (PDR). Neovascularization typically begins within the retina and on the optic nerve head but can eventually infiltrate the iris and angle. Tractional retinal detachment (TRD) and neovascular glaucoma are the most advanced complications and can cause severe, irreversible vision loss.
Diabetic macular edema (DME) is characterized by vascular leakage that results in intraretinal fluid cysts and retinal thickening that may be accompanied by spillover of subfoveal fluid in severe cases. This occurs as increased vasopermeability leads to breakdown of the inner blood-retinal barrier and exudation.3 While it is more likely as DR severity progresses, DME can occur at any stage of retinopathy and is the most common cause of reduced vision in diabetes.4
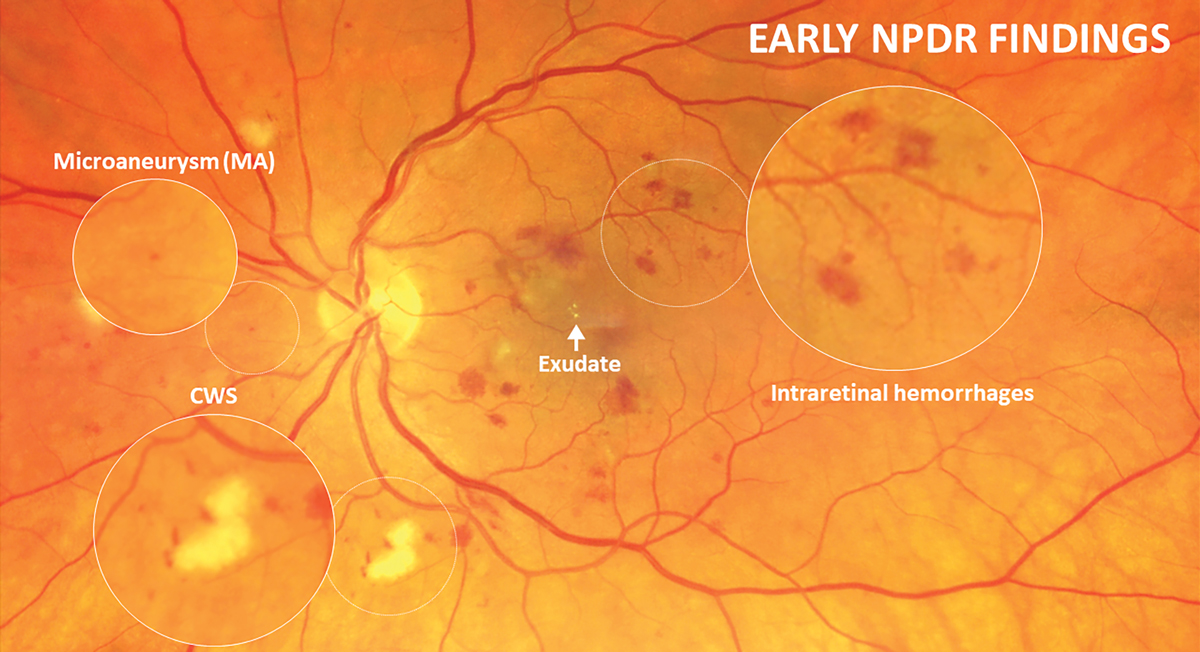 |
With the updated ICDR guidelines, mild retinopathy is classified as microaneurysms only and the presence of intraretinal hemorrhages is indicative of at least moderate NPDR stage. Click image to enlarge. |
An Update on DR Staging
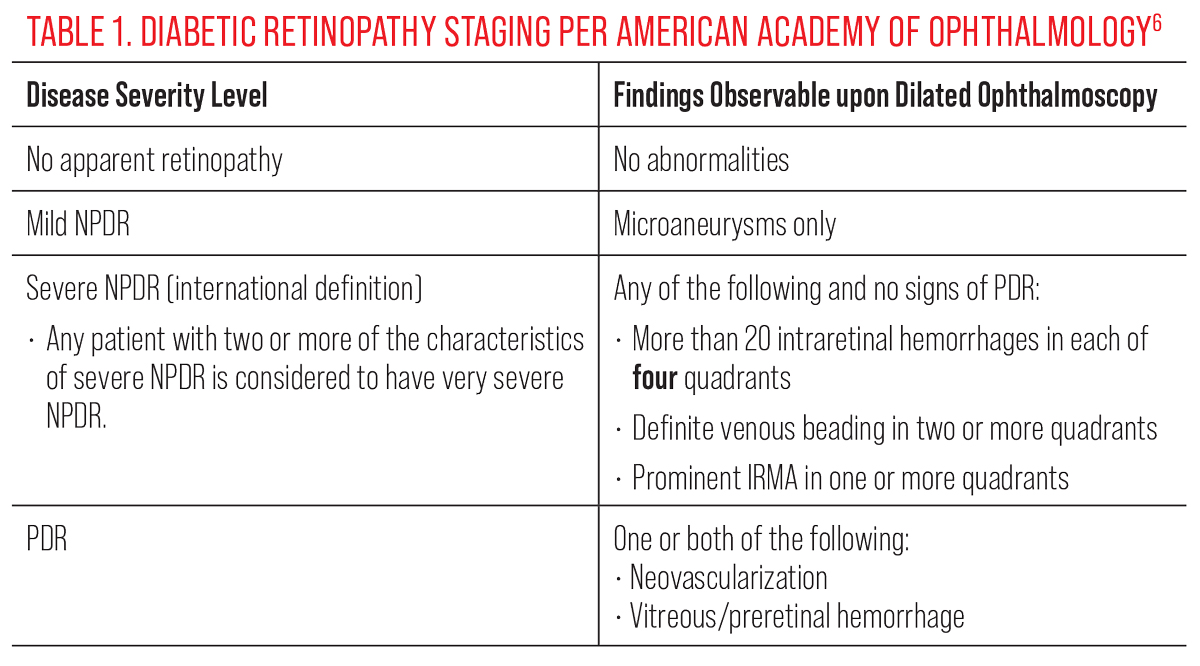
Nonproliferative diabetic retinopathy (NPDR) and PDR are divided into stages based upon evidence of progressing severity to provide risk assessment and guidance on clinical management. These grading scales have changed over time to reflect advances in knowledge and understanding of diabetic eye disease.
The Early Treatment of Diabetic Retinopathy Study (ETDRS) provided the most widely accepted classification system for many years.5 However, this dates back to 1991 and its applicability today suffers as a result. In 2019, the American Academy of Ophthalmology (AAOph) adjusted its Diabetic Retinopathy Preferred Practice Pattern to follow guidelines of another commonly used classification system, the International Clinical Diabetic Retinopathy (ICDR) Severity Scale.6 Although these grading scales have significant areas of overlap, there are some notable distinctions. The most significant difference between the two is the differentiation between mild vs. moderate NPDR.
Using the ETDRS classification, mild NPDR was characterized by microaneurysms and hemorrhaging less than that in that study’s Standard Photo 2A—a 30° color fundus photo can be used for DR comparison. With the updated ICDR guidelines, mild retinopathy is classified by microaneurysms only, and the presence of intraretinal hemorrhages is indicative of at least moderate NPDR stage. Severe NPDR continues to be defined by one factor of the 4-2-1 rule: four quadrants of severe intraretinal hemorrhaging (approximately 20 hemorrhages per quadrant), at least two quadrants of definite venous beading and at least one quadrant of IRMA. Very severe NPDR is associated with a higher risk of proliferative conversion and is defined as the presence of two or more factors of the 4-2-1 rule. Amongst eyes with severe and very severe NPDR, the risk for conversion to PDR within one year’s time is 50% and 75%, respectively.7,8
PDR is defined by the presence of new vessels with or without hemorrhaging into the vitreous or preretinal/subhyaloid space. Neovascularization in PDR is preretinal and located on top of the retina. It can be classified as neovascularization of the disc (NVD, on or within one disc diameter from the disc margin) or neovascularization of the retina elsewhere (NVE). These are weak and fragile vessels that often grow on the posterior hyaloid membrane, much like ivy grows on the side of a house.
PDR can be divided into active and inactive forms. Preretinal tissue in active PDR is still vascularized and blood can be seen within the small, fine vessels of the membrane, while the preretinal tissue of inactive PDR is mostly fibrotic, avascular and opaque in appearance.
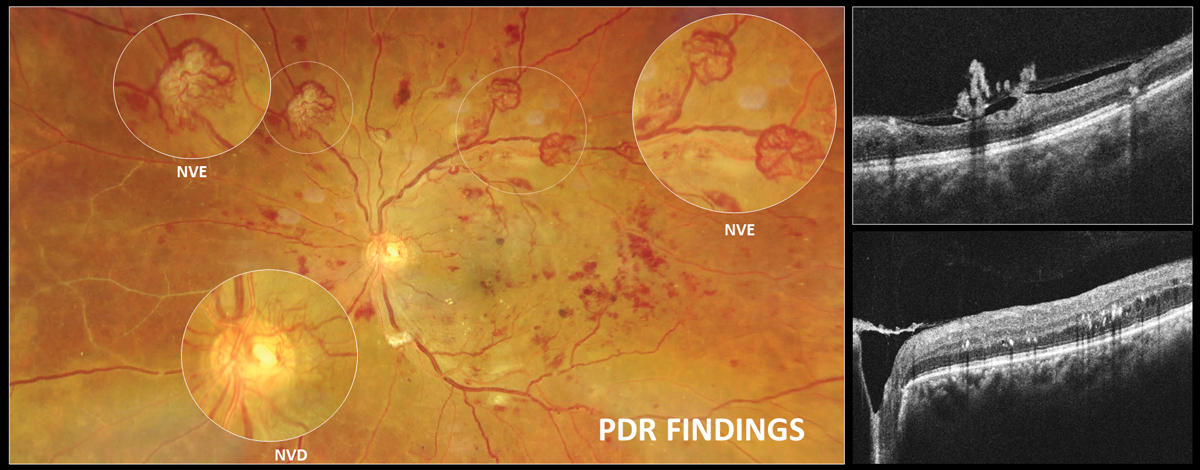 |
|
Neovascularization in PDR is preretinal and located on top of the retina. It can be classified as neovascularization of the disc (NVD, on or within one disc diameter from the disc margin) or neovascularization of the retina elsewhere (NVE). Click image to enlarge. |
With OCT angiography (OCT-A) there is regression of the lacy, looping capillaries on the fringe of the membrane as neovascularization becomes inactive; however, if the membrane is sizeable, the clinician may observe that larger, mature, feeder-looking vessels remain despite adequate treatment.
Active PDR can then further be subclassified as high risk or low risk. High-risk PDR is defined by the presence of NVD larger than one‐fourth disc area, a smaller area of NVD accompanied by vitreous or preretinal hemorrhage or NVE at least one-half disc area in size with vitreous or preretinal hemorrhage.6,8 Although any degree of PDR should be referred to a specialist and is usually treated, distinguishing high from low risk is important for two main reasons: (1) the 2019 American Optometric Association (AOA) Clinical Practice Guidelines recommend more urgent referral of high-risk PDR (within 24 to 48 hours vs. two to four weeks for low-risk) and (2) treatment, usually panretinal laser photocoagulation (PRP), is recommended for high-risk PDR since the Diabetic Retinopathy Study (DRS) found that there was an approximate 50% risk of severe vision loss within five years if high-risk PDR was left untreated.6,9,10
Additionally, anti-VEGF therapy to slow progression of PDR has recently gained traction and can decrease risk of complications from PDR such as TRDs and development of new macular edema. Further, the use of intravitreal anti-VEGF before or in addition to PRP has been demonstrated to be more effective than just PRP alone in high-risk PDR patients.11,12
Although the AAOph has adopted the ICDR system, it has not updated its practice guidelines similarly; the 2019 AOA Clinical Practice Guidelines continue to refer to the ETDRS scale DR grading criteria.9 These inconsistencies may lead to confusion if the grading scale used is not explicitly defined.
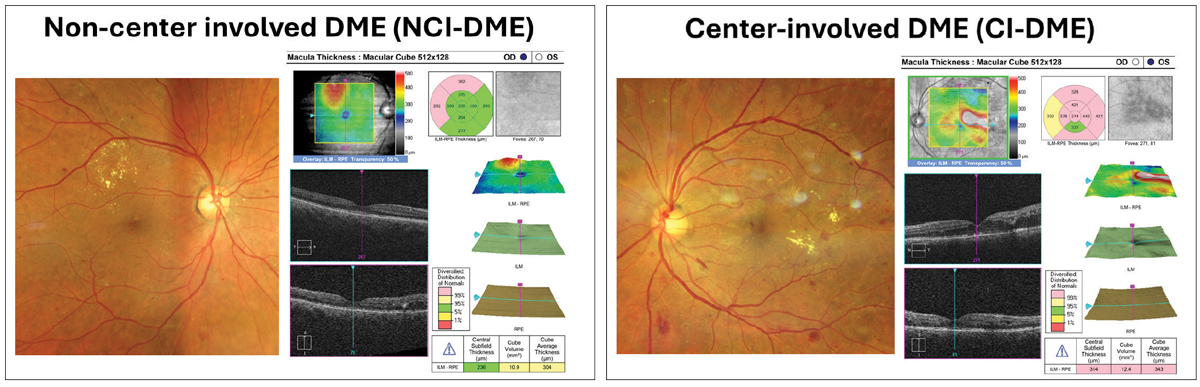
Historically, the ETDRS also coined the term “clinically significant macular edema” (CSME). This was defined as either retinal thickening within the central 500µm of the macula, hard exudates within 500µm of the center of the macula with adjacent thickening or zones of retinal thickening one disc diameter or larger within one disc diameter of the fovea.5,9 With the advent of OCT, DME is now more commonly described as either center involved (CI-DME) or non-center involved (NCI-DME). CI-DME is defined as thickening within the central 1mm diameter or “center subfield zone.”6 Staging retinopathy and DME offers an organized approach for management based upon risk of progression and ultimately, risk of vision loss.
 |
| Click table to enlarge. |
The OD’s Role
Proper management of patients with diabetes requires a multidisciplinary approach. With DR being the most common microvascular complication of the disease, optometric physicians play an integral part on this team.13
The American Diabetes Association (ADA) and AOA recommend a baseline comprehensive eye exam and follow-up examinations for patients with type 1 diabetes. The first follow-up should not exceed three to five years after diagnosis, and patients should be followed at least annually thereafter.9 Those with type 2 diabetes should be evaluated at time of diagnosis since diabetes may have gone undetected for years prior, making it challenging to determine the exact duration of the disease.9 Follow-up interval depends upon the level of retinopathy present and analysis of other risk factors for progression, such as degree of glycemic control and duration of the disease. Those without retinopathy or those with only mild retinopathy can be monitored annually assuming no DME is present.
There can be significant variability of presentation within the category of moderate NPDR, especially with the shift to the ICDR classification system. Milder cases of moderate NPDR with minimal retinopathy lesions can be monitored every nine to 12 months. However, as signs approach the more severe end of the moderate NPDR spectrum, a six-month follow-up interval may be more appropriate. Referral to a retina specialist should be considered for patients with severe NPDR even in the absence of DME, and patients should be monitored closely, every three to four months.6,9 When considering whether or not to refer a patient with severe NPDR, factors favoring referral include noncompliance, documented rapid progression, absence of complete posterior vitreous detachment (PVD), monocular status and presence of concurrent DME or multiple risk factors for progression such as poor glycemic control. Provider comfort level and in-office imaging availability also should be contemplated.
Electrophysiology, specifically electroretinogram (ERG) testing, is an additional tool that can aid in risk analysis for DR progression. ERG can provide a quantitative functional assessment of the retina that can uncover deficits before they become structurally evident. Patients with abnormal functional testing on ERG generally require earlier intervention than those with low-risk ERG results. In some cases, functional deficits can precede visible structural changes and may lead to closer monitoring and earlier referral.14
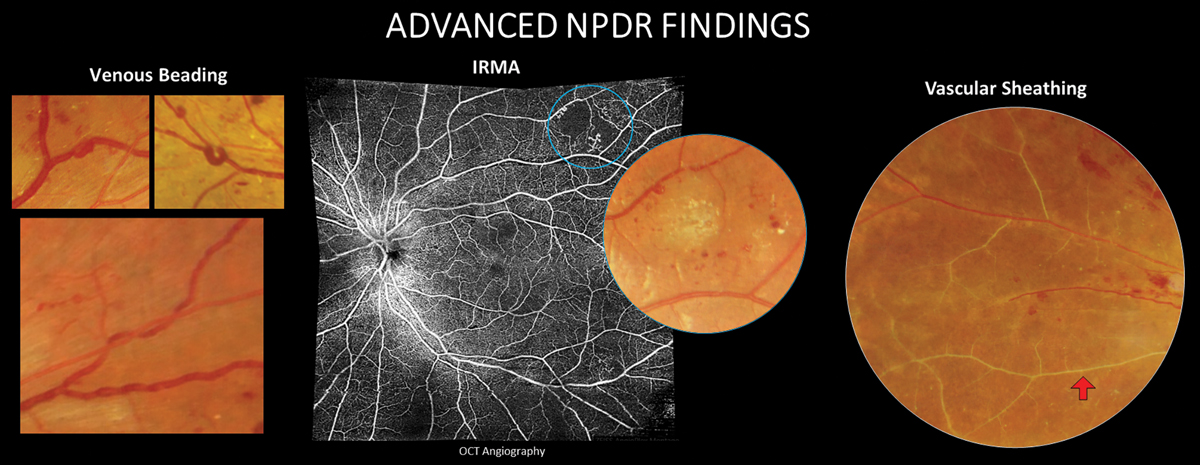 |
|
More severe NPDR findings that increase the risk for future conversion to PDR. Click image to enlarge. |
Observation is the current standard of care for cases of severe NPDR; however, some studies support beneficial outcomes of treatment at this stage, especially for those meeting the criteria of very severe NPDR.5,15,16 A referral to a retina specialist is also warranted for cases of active and previously untreated proliferative disease. As previously mentioned, the 2019 AOA Clinical Practice Guidelines recommend more urgent referral of high-risk PDR (24 to 48 hours for high-risk vs. two to four weeks for low-risk).9 Although treatment of low-risk PDR may or may not be immediately initiated (deferred until it reaches high risk), care with ophthalmology should be established. Due to the high risk for severe vision loss, the AAOph recommends treatment, usually PRP, in cases of active high-risk PDR.6
The presence of DME can have a significant impact on referral timeline. As a general rule, the optometric physician should consider referring DME at any stage, especially if it is center involved, causing reduced best-corrected visual acuity (BCVA) or is progressing. The 2019 AOA Clinical Practice Guidelines recommends consultation within two to four weeks when CI-DME is present.9 Of note, the results of the Diabetic Retinopathy Clinical Research (DRCR) Network Protocol V suggested that close observation until acuity declines or DME worsens may be a reasonable management option for eyes with CI-DME and BCVA of 20/25 or better.17 This study found that vision at two years was no different whether eyes with CI-DME and good acuity were immediately treated with aflibercept, macular laser or if treatment was withheld until acuity worsened.17 Patients with DME should be followed every two to four months.6,9,18
Refer patients with anterior segment neovascularization urgently, as prompt treatment prior to formation of complete synechial angle closure may spare an eye from surgical glaucoma procedures. Lastly, it may be beneficial to obtain consultation when the stage of retinopathy is uncertain due to media opacity or poor patient cooperation.
When patients with diabetes are examined, results should be communicated to the physician managing the diabetes (e.g., primary care physician, endocrinologist). This written communication should include the stage of retinopathy as well as the presence/absence of DME, encourage individualized glycemic control and other comorbid systemic risk factors such as hypertension/serum lipids and state the recommended follow-up.6,9,19 Recent evidence suggests that episodes of hypoglycemia can contribute to worsening diabetic eye disease via upregulation of VEGF. Take care to avoid even transient periods of low blood sugar.20
Eyecare providers have an obligation to the public to provide access to care for all patients—even those in rural areas— and education efforts should be made to ensure that the public is aware that diabetes can affect the eyes, emphasizing the importance of at least yearly eye examinations and that vision loss is preventable with early detection.21
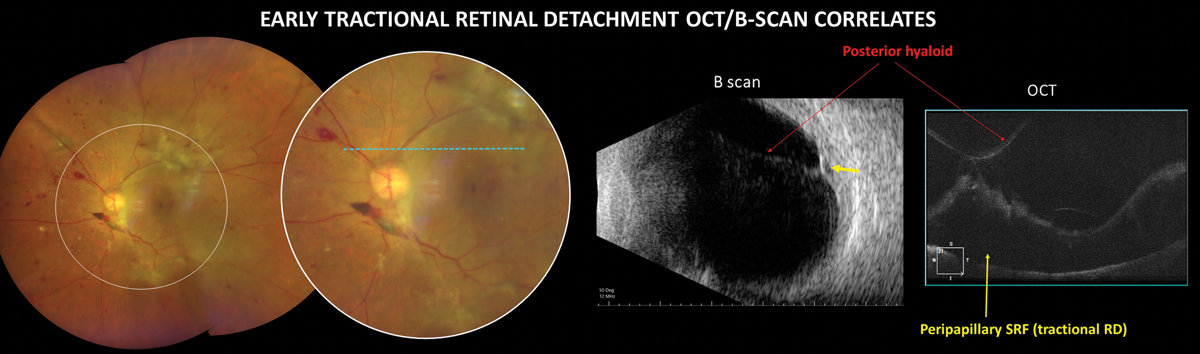 |
|
TRD in PDR is an advanced complication that can cause severe, irreversible vision loss. Click image to enlarge. |
Examination Essentials
Exams for patients with diabetes should be multifaceted and emphasize certain aspects. First, a careful history should be acquired. Document duration of DM, some indication of glycemic control (HbA1c and/or fasting blood glucose level, glucose time in range) and medications. Visual acuity, entrance test, slit lamp exam and intraocular pressure (IOP) should be obtained, as in a standard eye exam. A magnified slit lamp examination of the entire iris (lids retracted as needed) should be done prior to dilation. If any neovascularization of the iris is present or IOP is elevated, perform gonioscopy to inspect for neovascularization of the angle.
After dilation, perform a thorough funduscopic exam of the posterior pole and the periphery. Vitreous and preretinal hemorrhages may be subtle in PDR, and it is important to always examine the inferior peripheral fundus carefully to look for subtle vitreous blood that has settled with gravity.
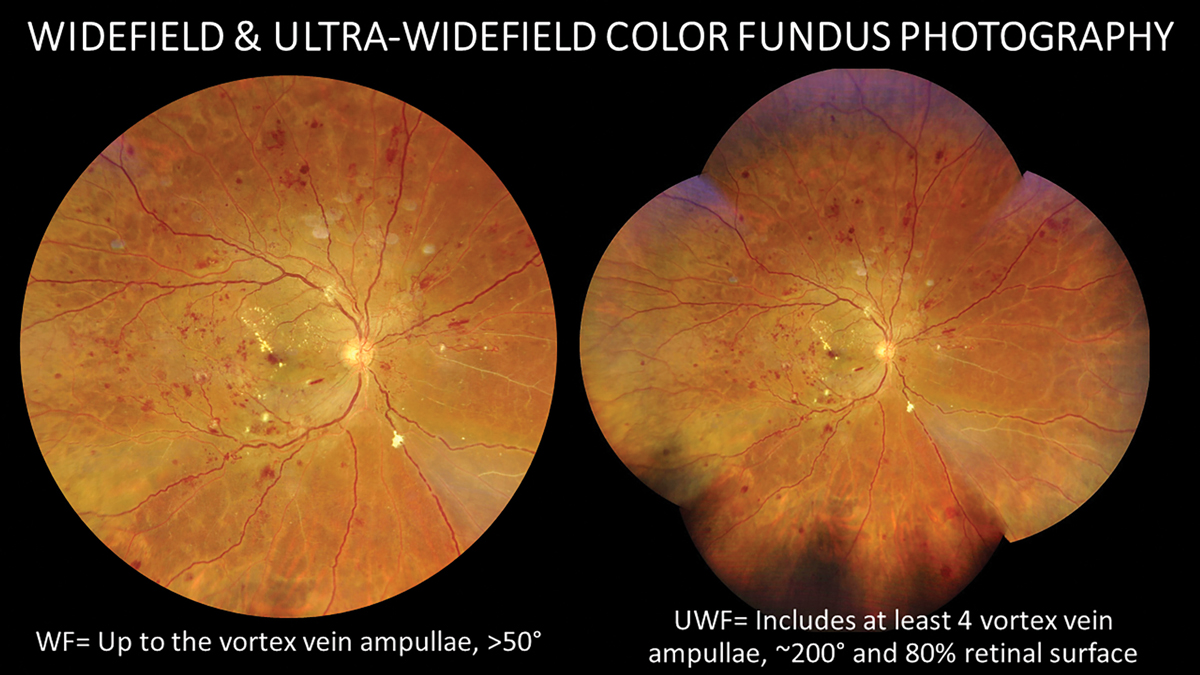
Although not required, certain ancillary tests provide great benefit in diagnosis and management. Multimodal imaging technologies often help highlight subtle vascular abnormalities and results in more accurate retinopathy staging and increased exam efficiency. There is significant debate regarding the use of fundus photography as a replacement for traditional dilated eye exams. Multiple studies have shown that inspection of fundus photos may actually be superior to ophthalmoscopy for identification of some lesions such as microaneurysms, small intraretinal hemorrhages, IRMA and subtle neovascularization.22,23 However, at this point in time, imaging is less than a perfect science. The presence of artifacts, lack of stereopsis and poor identification of peripheral lesions are just some reasons why imaging, including ultra-widefield techniques, is often not an adequate replacement for clinical examination.24 A traditional dilated fundus exam is still the standard of care for diabetes from a legal perspective, and examination of the retina via imaging alone is inadequate. Rather than substituting photography for comprehensive clinical examination of diabetic patients, use fundus imaging as an adjunctive tool in addition to traditional ophthalmoscopy.
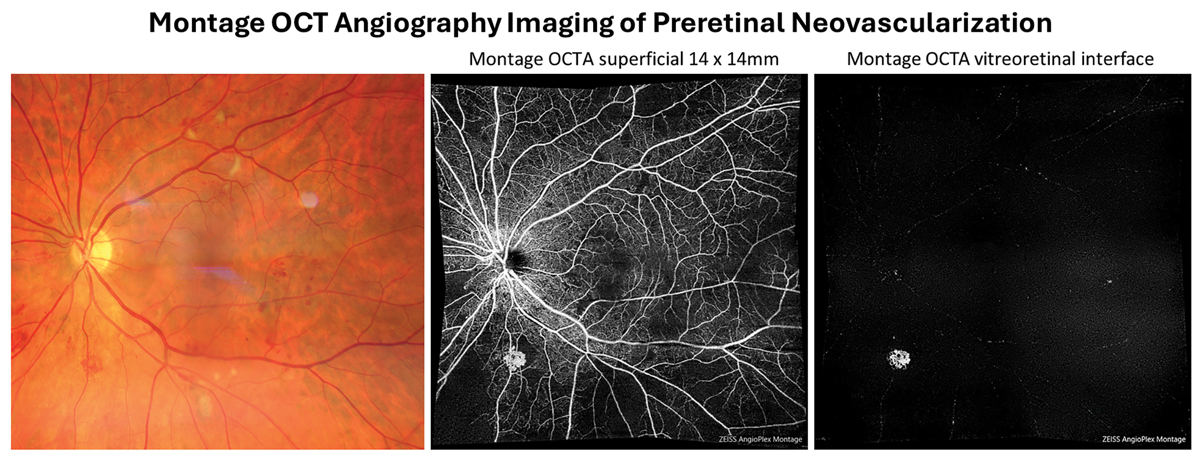 |
|
Preretinal neovascularizaton can be isolated out using the vitreous or vitreoretinal interface preset en-face display of OCT-A. This allows for the earliest detection of even subclinical PDR. Click image to enlarge. |
One of the most heavily used ancillary instruments in these eye exams is OCT. It has proven incredibly valuable in the diagnosis and management of DME, as it can quantify central macular thickness, identify anatomical location of fluid (including subclinical DME), closely monitor changes in macular edema and rule in or out other causes of reduced vision in patients with diabetes. Additionally, OCT is routinely used to monitor DME treatment efficacy. OCT, and especially OCT-A or fluorescein angiography, can also be used to confirm or deny the presence of neovascularization that can be difficult to differentiate from IRMA or other anomalous appearing vessels. Even structural OCT alone is an excellent tool that can identify preretinal tissue or vitreoretinal traction and monitor TRD.
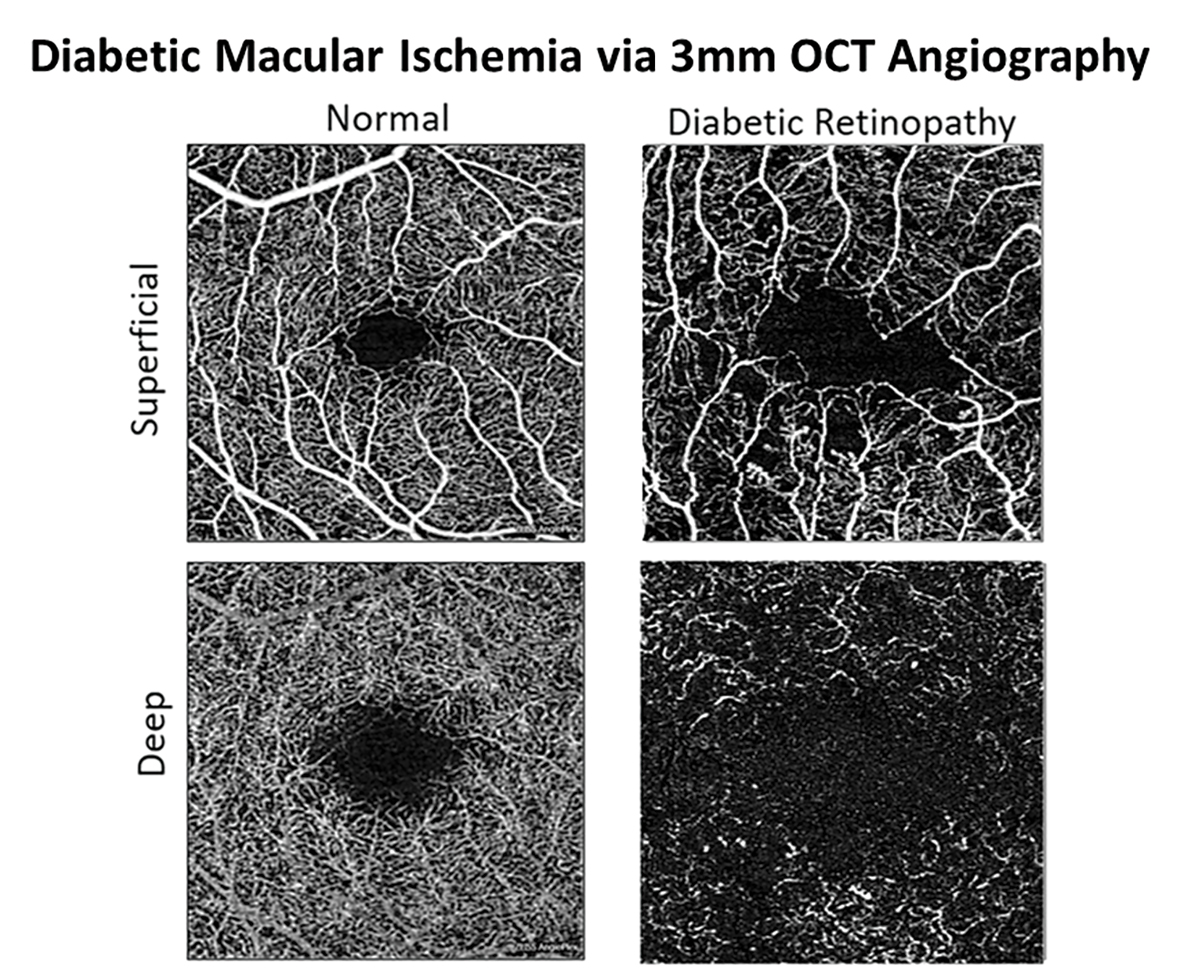
In recent years, development of OCT-A has provided a noninvasive method for optometric physicians to assess capillary nonperfusion, macular ischemia and neovascularization in-office. Previously, these findings could only be directly visualized via fluorescein angiography, requiring an ophthalmology referral in most states. Smaller scan sizes that maximize resolution, such as 3mm by 3mm, should be used to evaluate macular ischemia while wider field montage imaging, such as 14mm by 14mm, should be employed for estimating the degree of retinal nonperfusion and screening for proliferation. Neovascularization often occurs adjacent to areas of capillary nonperfusion. Incorporating imaging technologies allows for the earliest detection of even subclinical PDR.
B-scan ultrasonography may not be a heavily used tool in many optometry practices; however, its utility in cases of dense vitreous hemorrhage is unmatched. As previously discussed, DR with any vitreous or preretinal hemorrhage must be referred to a retina specialist for treatment. However, the presence of a TRD observed with B-scan increases the urgency of referral.
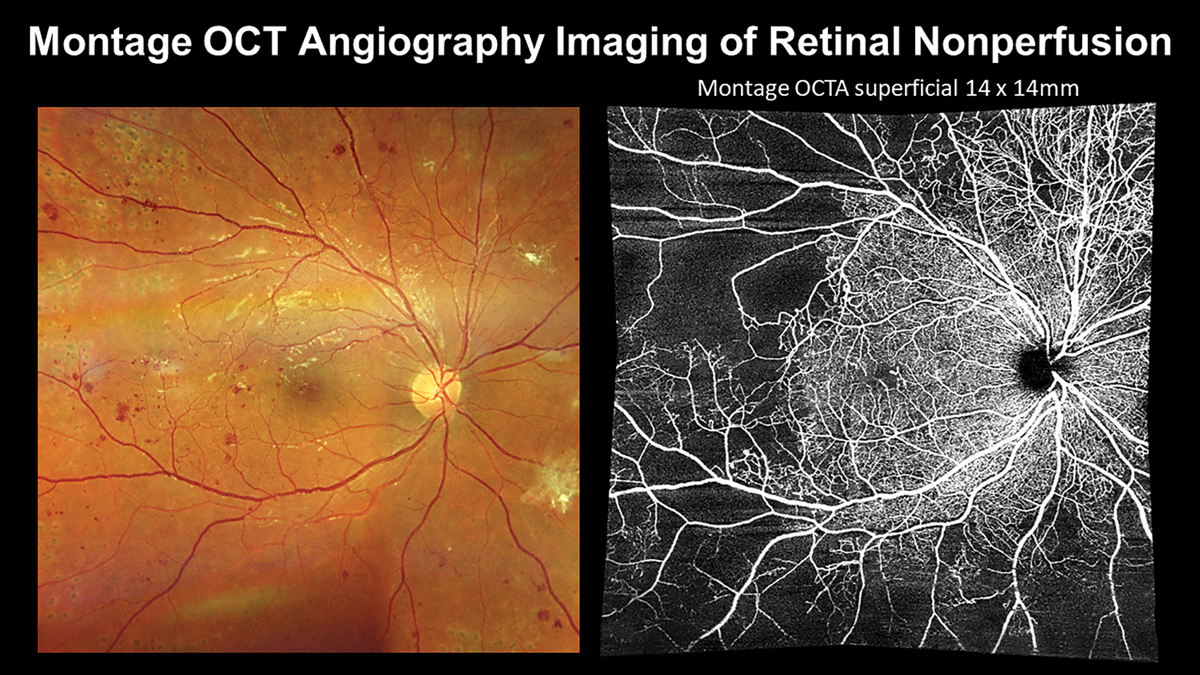 |
|
Incorporating montage OCT-A enables visualization of retinal nonperfusion that may be nearly invisible with ophthalmoscopy or color photography alone. Click image to enlarge. |
Patient Education
It is essential that patients are informed that at least annual eye exams are necessary even if they are asymptomatic. It must be conveyed that early detection and timely treatment of DR are essential for best visual outcomes and prevention of sight-threatening complications. Especially in those with severe NPDR or PDR, optometrists should emphasize specific symptoms of vitreous hemorrhage and retinal detachment so that patients are aware they need to be seen as soon as possible upon onset.
To adequately educate their patients, optometrists should be aware of risk factors for progression of retinopathy. Disease duration is a well-known risk factor for DR development and progression. This is especially true for those with type 1 diabetes. After 15 years of disease duration, 80% of patients with type 1 DM will have some degree of retinopathy.25 Intuitively, elevated blood glucose levels and HbA1c values, as well as less glucose time in range assessed by continuous glucose monitoring devices, are also associated with higher rates of retinopathy.
An HbA1c ≤7% is recommended for most patients, depending on expected lifespan, comorbidities and cognitive status.26 Control of blood pressure, lipids and management of comorbidities, such as sleep apnea, all reduce risk of progression.27 Additionally, awareness of high-risk demographics is valuable for adequate patient education. Notably, rates of diabetes are significantly higher among Native American, Hispanic and African American populations, and these patients have higher risk of vision loss.6
Outline the risk of rapid progression carefully for women with diabetes who become pregnant. Changes in metabolic control during pregnancy are thought to contribute to increased risk of progression to severe retinopathy.28 The AOA recommends a comprehensive eye exam prior to pregnancy, during the first trimester and more often thereafter as indicated by severity of retinopathy. Interestingly, there does not appear to be a significantly increased risk of retinopathy for those who develop gestational diabetes.29
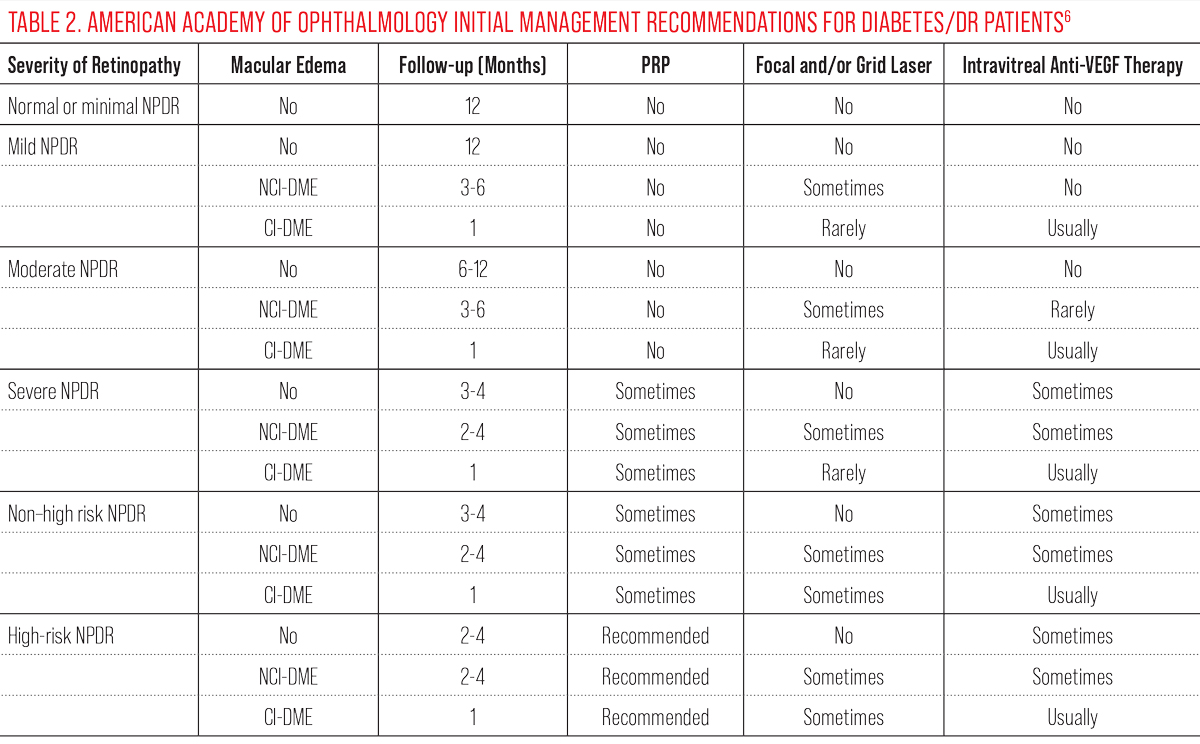 |
| Click table to enlarge. |
DR in the Ozempic Era
Glucagon-like peptide-1 (GLP-1) receptor analogs are a newer, highly effective category of medication for diabetes management, recently skyrocketing in popularity. GLP-1 drugs such as semaglutide (Ozempic and Wegovy, both Novo Nordisk), as well as tirzepatide (Mounjaro, Eli Lilly), have risen to popularity because of their efficacy for glycemic control and weight loss promotion. With an increasing number of diabetic patients being prescribed these drugs, it is important that eyecare providers understand the implications.
Although improved glycemic control is encouraged for promotion of long-term positive outcomes, a transient worsening of retinopathy may be seen initially. The pathophysiology of this phenomenon is not well understood but is likely related to VEGF expression, reactive oxygen species production and breakdown of the blood-retinal barrier.30
A post-hoc review of the Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes 6 (SUSTAIN 6) clinical trial compared DR in patients treated with semaglutide vs. placebo.31 In general, patients treated with semaglutide had a higher incidence of DR progression compared with the placebo group. It is presumed that this is due to the rapid decrease in HbA1c during the first 16 weeks of treatment.32 This study found the highest rates of DR complications in those with an A1c reduction of 1.5%.33 Interestingly, there was no difference between the treated and placebo groups in those without retinopathy at baseline. Rather, progression was primarily observed in patients with NPDR and PDR upon initiation of treatment. Those with pre-existing retinopathy who were using insulin were at significantly higher risk of developing worsening retinopathy. However, it is not clear if this is due to interaction with insulin or because these patients generally have had a longer duration of diabetes and higher baseline A1c.
As more patients are started on GLP-1 analogs, optometrists should be aware of the frequency of paradoxical worsening of retinopathy that occurs with rapidly improved glycemic control. Some sources suggest a baseline exam prior to initiation of treatment.33 Additionally, more frequent follow-up of high-risk patients, such as those with existing severe retinopathy and insulin use, may be indicated during the first few months of treatment until glucose levels have stabilized.
 |
|
Staging of retinopathy and DME offers an organized approach for management based upon risk of progression and ultimately, risk of vision loss. CI-DME is defined as thickening within the central subfield zone (1mm in diameter). Click image to enlarge. |
Management Considerations
At each stage of DR, there are many findings and options to discuss with patients.
NPDR. Oral fenofibrate and vitamin supplementation may be considered in certain patients with mild to moderate NPDR in an effort to slow progression and improve visual function.
Fenofibrate is an older dyslipidemia medication that is a safe and inexpensive fibric acid derivative. It is considered off-label for treating DR in the US but is approved for this purpose in Australia and Singapore.34 The typical dose when treating DR is 160mg per day; however, caution should be exercised in patients with kidney disease and a lower dose of approximately 54mg should be used.35,36
Two very large randomized controlled trials (FIELD and ACCORD) have shown that fenofibrate, when used as an adjunctive treatment to standard of care DR therapies, can slow progression of pre-existing DR and reduce the need for treatment of DME and proliferative DR in patients with type 2 diabetes.37-39 The DRCR Network is currently recruiting for Protocol AF (Fenofibrate for Prevention of DR Worsening), which is enrolling patients with mild to moderately severe NPDR and no CI-DME at baseline. Results are expected in 2029.36
In clinical practice, fenofibrate should be considered in patients with type 2 diabetes, mild to moderate NPDR and normal kidney function.34,35,40 It may be particularly beneficial among those with moderate to very high cardiovascular risk factors (hypertension, dyslipidemia, previous history of cardiovascular events) and at high risk for DR progression.35
Another strategy aimed at DR prevention is nutritional supplementation and medical food therapies. We know that many patients with DR have concurrent vitamin and mineral deficiencies that fuel retinal microvascular damage and inflammation.41 Similar to use of fenofibrate, supplementation with vitamins, minerals and nutraceuticals is intended to complement current standard of care treatments, not replace them. The goals of supplementation in DR are to decrease inflammatory mediators, reduce oxidative stress, support retinal metabolism and promote microvascular health.41
 |
|
A traditional dilated fundus exam is still the standard of care for diabetes retinal assessment from a legal perspective, but widefield imaging is a highly valuable adjunctive tool. Click image to enlarge. |
Supplement components of interest include lutein and zeaxanthin, L-methylfolate, N-acetylcysteine, vitamin E, vitamin D, vitamin C, vitamins B1, B2, B6 and B12 (methylcobalamin) and alpha-lipoic acid.41 One study demonstrated that carotenoid containing nutritional supplementation in diabetic rats helped prevent capillary cell apoptosis and maintain normal retinal function.42 Furthermore, the Diabetes Visual Function Supplement Study (DiVFuSS) evaluated the effectiveness of a commercially available vitamin supplement (EyePromise DVS) on functional vision aspects in 67 patients with diabetes and either no or mild-moderate stage nonproliferative DR.43 Patients were randomized to either six months of DVS supplementation or placebo. Subjects on DVS had significantly better visual function performance on contrast sensitivity, color discrimination and 5-2 macular threshold perimetry testing.43 Another commercially available medical food, Ocufolin (Global Healthcare Focus), has demonstrated an improvement in retinal microcirculation in eyes with mild DR among patients with methylenetetrahydrofolate reductase polymorphisms.44
Early intravitreal anti-VEGF therapy may be considered in eyes with moderately severe to severe NPDR in an effort to improve retinopathy stage and/or prevent vision threatening complications, although practice patterns and sentiments in this regard vary greatly amongst retinal specialists.15,16 The DRCR Network Protocol W study set out to evaluate the potential benefits of periodic anti-VEGF therapy in preventing vision-threatening complications such as PDR and CI-DME.16 This study included eyes with moderate to severe NPDR lacking CI-DME and randomized them to either immediate periodic aflibercept injections or observation until CI-DME or high-risk PDR developed. The four-year results showed that while early anti-VEGF therapy did reduce the risk of developing CI-DME and PDR significantly, change in visual acuity did not differ between the two groups.16 Therefore, the role of anti-VEGF therapy in severe NPDR management and its potential benefits continues to be debated.
PDR. Both PRP and anti-VEGF may be used to treat PDR; however, PRP is generally recommended in high-risk PDR.6 Although the DRCR Network Protocol S demonstrated that periodic ranibizumab therapy for PDR resulted in non-inferior acuity outcomes, less peripheral field loss and lower rates of vision-impairing DME development when compared with PRP, it is important to remember that anti-VEGF effects are temporary.45 Long-term studies demonstrate that poor follow-up compliance amongst PDR patients treated with anti-VEGF alone can result in visually devastating outcomes, and follow-up compliance can be a challenge in patients with diabetes who need to see multiple specialty providers of various disciplines and may be hospitalized more frequently.46 PRP may also be favored over anti-VEGF therapy in eyes with significant vitreoretinal traction since rapid neovascular regression may exacerbate this and promote TRD development/progression.47
 |
|
Development of OCT-A has provided a noninvasive method for optometrists to assess capillary nonperfusion, macular ischemia and neovascularization in-office. Click image to enlarge. |
If concurrent high-risk PDR and CI-DME are present, treatment usually begins with anti-VEGF and then delayed PRP is performed, since PRP may initially worsen DME.6 Regression of neovascularization following PRP alone should begin within four to six weeks and supplemental PRP, anti-VEGF therapy or vitrectomy may be management options should PRP result in inadequate neovascular involution.6
Vitreous hemorrhage and TRD. Vitreous hemorrhage may be initially observed when PRP has already been performed and B-scan ultrasonography confirms no TRD is present. Intravitreal anti-VEGF may speed resolution of vitreous hemorrhage and is often used when vitreoretinal traction is limited. Educate patients to limit physical activity, especially heavy lifting and Valsalva maneuvers, sleep upright if possible and avoid bending the head below the waist. Vitrectomy may be considered in cases of macular-threatening or -involving TRD, non-clearing or recurrent vitreous hemorrhage, dense preretinal hemorrhage or fibrotic tissue covers the macula, florid PDR with very large areas of neovascularization is present or DME with a vitreous tractional component exists.6
DME. First-line therapy for CI-DME involves anti-VEGF therapy, and newer generation agents such as faricimab and high-dose 8mg aflibercept may provide extended duration of action allowing for less frequent injections and reduced treatment burden.6,9,47-49 Macular photocoagulation remains a viable treatment option for non-center-involved DME, especially when focal areas of noncentral leakage are present.6 Intravitreal corticosteroid injections and sustained-release implants may be employed when DME is diffuse or unresponsive to anti-VEGF therapies, especially in pseudophakic eyes.50
Imaging Applications in DRThis is not an exhaustive list, but we do find these three tools ideally suited to each of the following responsibilities. OCT
ОСТ-А
Widefield Imaging
|
Takeaways
With rates of diabetes skyrocketing, optometrists are and will continue to be a valuable asset to the multidisciplinary diabetes care team. As our understanding of DR deepens, our care must reflect these changes and advances. By staying up to date on guidelines, diagnostics aids, risk factors and management standards, optometrists can continue to offer quality care to patients with diabetes.
Dr. Tarka is a resident at the Oklahoma College of Optometry at Northeastern State University.
Dr. Majcher is a professor and the director of residency programs at the Oklahoma College of Optometry at Northeastern State University. She is a fellow of the American Association of Optometry and the Optometric Retina Society. She is a paid speaker and consultant for Regeneron Pharmaceuticals and Carl Zeiss Meditec. She is also a paid consultant for Topcon as well as Ocuterra and has received non-financial support from Roche.
1. Bryan S, Afful J, Carroll M, et al. NHSR 158 national health and nutrition examination survey 2017–March 2020 pre-pandemic data files. National Health Statistics Reports. stacks.cdc.gov/view/cdc/106273. June 14, 2021. Accessed April 23, 2024. 2. Tan TE, Wong TY. Diabetic retinopathy: looking forward to 2030. Front Endocrinol (Lausanne). 2023;13:1077669. 3. Romero-Aroca P, Baget-Bernaldiz M, Pareja-Rios A, et al. Diabetic macular edema pathophysiology: vasogenic vs. inflammatory. J Diabetes Res. 2016;2016:2156273. 4. Musat O, Cernat C, Labib M, et al. Diabetic macular edema. Rom J Ophthalmol. 2015;59(3):133-6. 5. Early Treatment Diabetic Retinopathy Study Research Group. Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Ophthalmology. 1991;98(5 Suppl):766-85. 6. Flaxel CJ, Adelman RA, Bailey ST, et al. Diabetic retinopathy preferred practice pattern. Ophthalmology 2020;127(1):P66-145. 7. Early Treatment Diabetic Retinopathy Study Research Group. Fundus photographic risk factors for progression of diabetic retinopathy: ETDRS report number 12. Ophthalmology. 1991;98(5):823-33. 8. Perais J, Agarwal R, Evans JR, et al. Prognostic factors for the development and progression of proliferative diabetic retinopathy in people with diabetic retinopathy. Cochrane Database Syst Rev. 2023 Feb 22;2(2):CD013775. 9. American Optometric Association. Evidence-based clinical practice guideline: eye care of the patient with diabetes mellitus. 2nd ed. www.aoa.org/practice/clinical-guidelines/clinical-practice-guidelines?sso=y. Accessed April 23, 2024. 10. The Diabetic Retinopathy Study Research Group. Photocoagulation treatment of proliferative diabetic retinopathy. Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS Report Number 8. Ophthalmology. 1981;88(7):583-600. 11. Bahr TA, Bakri, SJ. Update on the management of diabetic retinopathy: anti-VEGF agents for the prevention of complications and progression of nonproliferative and proliferative retinopathy. Life. 2023;13(5):1098. 12. Osaadon P, Fagan XJ, Lifshitz T, et al. A review of anti-VEGF agents for proliferative diabetic retinopathy. Eye. 2014;28(5): 510-20. 13. Shukla UV, Tripathy K. Diabetic retinopathy. StatPearls. Treasure Island (FL): StatPearls Publishing. www.ncbi.nlm.nih.gov/books/NBK560805/. Last updated August 25, 2023. Accessed April 23, 2024. 14. Brigell MG, Chiang B, Maa AY, Davis CQ. Enhancing risk assessment in patients with diabetic retinopathy by combining measures of retinal function and structure. Transl Vis Sci Technol. 2020;9(9):40. 15. Brown DM, Wykoff CC, Boyer D, et al. Evaluation of intravitreal aflibercept for the treatment of severe nonproliferative diabetic retinopathy. JAMA Ophthalmol. 2021;139(9):946-55. 16. Maturi RK, Glassman AR, Josic K, et al. Four-year visual outcomes in the Protocol W randomized trial of intravitreous aflibercept for prevention of vision-threatening complications of diabetic retinopathy. JAMA. 2023;329(5):376-85. 17. Baker CW, Glassman AR, Beaulieu WT, et al; DRCR Retina Network. Effect of initial management with aflibercept vs. laser photocoagulation vs. observation on vision loss among patients with diabetic macular edema involving the center of the macula and good visual acuity: a randomized clinical trial. JAMA. 2019;321(19):1880-94. 18. Al Qassimi N, Kozak I, Al Karam M, et al. Management of diabetic macular edema: guidelines from the Emirates Society of Ophthalmology. Ophthalmol Ther. 2022;11(5):1937-50. 19. Storey PP, Murchison AP, Pizzi LT, et al. Impact of physician communication on diabetic eye examination adherence: Results from a retrospective cohort analysis. Retina. 2016;36(1):20-7. 20. Drinkwater JJ, Davis TME, Turner AW, et al. Incidence and determinants of intraocular lens implantation in type 2 diabetes: the Fremantle Diabetes Study Phase II. Diabetes Care. 2019;42(2):288-96. 21. Piyasena MMPN, Murthy GVS, Yip JLY, et al. Systematic review on barriers and enablers for access to diabetic retinopathy screening services in different income settings. PLoS One. 2019;14(4):e0198979. 22. Brown K, Sewell JM, Trempe C, et al. Comparison of image-assisted vs. traditional fundus examination. Eye Brain. 2013;5:1-8. 23. Cavallerano JD, Aiello LP, Cavallerano AA, et al. Nonmydriatic digital imaging alternative for annual retinal examination in persons with previously documented no or mild diabetic retinopathy. Am J Ophthalmol. 2005;140(4):667-73. 24. Kornberg DL, Klufas MA, Yannuzzi NA, et al. Clinical utility of ultra-widefield imaging with the optos optomap compared with indirect ophthalmoscopy in the setting of non-traumatic rhegmatogenous retinal detachment. Semin Ophthalmol. 2016;31(5):505-12. 25. Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol. 1984;102(4):520-6. 26. Solomon SD, Chew E, Duh EJ, et al. Diabetic retinopathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(3):412-8. 27. Simonson M, Li Y, Zhu B, et al. Multidimensional sleep health and diabetic retinopathy: systematic review and meta-analysis. Sleep Med Rev. 2024;74:101891. 28. Chew EY, Mills JL, Metzger BE, et al. Metabolic control and progression of retinopathy: the diabetes in early pregnancy study. Diabetes Care. 1995;18(5):631-7. 29. Chandrasekaran P, Madanagopalan V, Narayanan R. Diabetic retinopathy in pregnancy - a review. Indian J Ophthalmol. 2021;69(11):3015-25. 30. Bain SC, Klufas MA, Ho A, Matthews DR. Worsening of diabetic retinopathy with rapid improvement in systemic glucose control: a review. Diabetes Obes Metab. 2018;21(30):454-66. 31. Vilsbøll T, Bain SC, Leiter LA, et al. Semaglutide, reduction in glycated haemoglobin and the risk of diabetic retinopathy. Diabetes Obes Metab. 2018;20(4):889-97. 32. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834-44. 33. Berkovic MC, Strollo F. Semaglutide-eye-catching results. World J Diabetes. 2023;14(4):424-34. 34. Stewart S. Fenofibrate for diabetic retinopathy. Asia Pac J Ophthalmol (Phila). 2018;7(6):422-6. 35. Ngah NF, Muhamad NA, Abdul Aziz RA, et al. Fenofibrate for the prevention of progression of non-proliferative diabetic retinopathy: review, consensus recommendations and guidance for clinical practice. Int J Ophthalmol. 2022;15(12):2001-8. 36. NCT04661358. Fenofibrate for prevention of DR worsening (Protocol AF). clinicaltrials.gov/study/NCT04661358. Last updated March 27, 2024. Accessed April 23, 2024. 37. Keech A, Simes RJ, Barter P, et al.; FIELD study investigators. Effects of long-term fenofibrate therapy on cardiovascular events in 9,795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366(9500):1849-61. 38. Keech AC, Mitchell P, Summanen PA, et al.; FIELD study investigators. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet. 2007;370(9600):1687-97. 39. Chew EY, Davis MD, Danis RP, et al. The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. Ophthalmology. 2014;121(12):2443-51. 40. Wong TY, Simó R, Mitchell P. Fenofibrate - a potential systemic treatment for diabetic retinopathy? Am J Ophthalmol. 2012;154(1):6-12. 41. Shi C, Wang P, Airen S, et al. Nutritional and medical food therapies for diabetic retinopathy. Eye Vis (Lond). 2020;7:33. 42. Kowluru RA, Zhong Q, Santos JM, et al. Beneficial effects of the nutritional supplements on the development of diabetic retinopathy. Nutr Metab (Lond). 2014;11(1):8. 43. Chous AP, Richer SP, Gerson JD, Kowluru RA. The Diabetes Visual Function Supplement Study (DiVFuSS). Br J Ophthalmol. 2016;100(2):227-34. 44. Liu Z, Jiang H, Townsend JH, Wang J. Improved retinal microcirculation in mild diabetic retinopathy patients carrying MTHFR polymorphisms who received the medical food, Ocufolin. Clin Ophthalmol. 2022;16:1497-504. 45. Gross JG, Glassman AR, Liu D, et al.; Diabetic Retinopathy Clinical Research Network. Five-year outcomes of panretinal photocoagulation vs. intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA Ophthalmol. 2018;136(10):1138-48. 46. Obeid A, Su D, Patel SN, et al. Outcomes of eyes lost to follow-up with proliferative diabetic retinopathy that received panretinal photocoagulation vs. intravitreal anti-vascular endothelial growth factor. Ophthalmology. 2019;126(3):407-13. 47. Wykoff CC, Abreu F, Adamis AP, et al. YOSEMITE and RHINE Investigators. Efficacy, durability and safety of intravitreal faricimab with extended dosing up to every 16 weeks in patients with diabetic macular oedema (YOSEMITE and RHINE): two randomized, double-masked, Phase III trials. Lancet. 2022;399(10326):741-55. 48. DME: extended dosing. Eylea. eyleahcp.us/s/eyleahd/dme-extended-dosing. Accessed April 23, 2024. 49. DME: vision outcomes. Eyelea. eyleahcp.us/s/eyleahd/dme-vision-outcomes. Accessed April 23, 2024. 50. Chawan-Saad J, Wu M, Wu A, Wu L. Corticosteroids for diabetic macular edema. Taiwan J Ophthalmol. 2019;9(4):233-242. |

