Transformative TechOptometrists today have more tools than ever at their disposal to help detect, treat and manage disease. To help you keep up with the constant innovation, the September 2024 issue of Review of Optometry—our 47th annual technology report—reviews the pros and cons of telehealth in optometry, new devices in eye care and the future of remote monitoring. Plus, find the results of our annual tech survey to discover which clinical instruments your peers are buying or desiring. Check out the other articles featured in this issue: |
Most primary care optometrists are well-equipped to serve any patient who walks in the door with the standard battery of tools and technology that make up the core pieces of an exam suite, but technological advancements are continuously creating new options to improve your existing clinical procedures or even add entirely new ones for those doctors looking to level up. From sophisticated imaging systems to cutting-edge diagnostic tools, a range of optometric devices have emerged that can enhance patient care and improve clinical outcomes.
This article will explore a diverse selection of optometric devices that are newer to the scene or mostly associated with different types of specialty care that may help you advance your skills in a particular sphere of care you want to develop. We’ll delve into their potential benefits and whether they might be a good fit for your specific needs. No single OD would conceivably want or need all of these, but as more doctors gravitate toward a particular niche or subspecialization to concentrate on, it’s worth looking at advanced tools.
By asking experts about the latest innovations and assessment of their practicality, we aim to provide insights on what these devices can offer for ODs who are currently kicking the tires on a new purchase.
Scleral Topography/Profilometry
One big success story in recent years has been resurgence of scleral contact lenses. Fitting sets do a nice job, but a scleral topographer allows doctors to assess exact curvature and surface characteristics—a crucial component of fitting sclerals with precision. Scleral topography, or scleral profilometry, helps design lenses that conform optimally to the eye’s unique surface, ensuring better lens alignment, stability and comfort.
“The promise of profilometry is reduced chair time to complete the specialty lens fitting. More information can be gathered regarding the ocular surface shape before any lens is manufactured,” notes Lindsay Sicks, OD, of the Illinois College of Optometry. “Highly customized geometry can be manufactured based on the scans obtained, so these devices can be very useful in patients who have failed with standard scleral lenses or who want a more streamlined fitting process.”
John Gelles, OD, who specializes in keratoconus and contact lenses at The Cornea and Laser Eye Institute of Teaneck, NJ, considers this device indispensable for his clinical practice. “Scleral profilometry can significantly improve your ability to fit complex eyes and helps you understand scleral shape on a higher level. The data allows you to predetermine the lens design that will work best for your patient,” he says, while also noting that using scleral profilometry does come with a learning curve.
There can be the misconception that the device will give you perfect lenses on one try and need no modifications, bypassing practitioner skill. However, that is not the case. “Rather,” Dr. Gelles explains, “think of these devices as giving you a better start point.”
While a scleral topographer can produce better-fitting lenses, you need good scans to do so, he explains. “Familiarizing yourself with the device and learning how to capture and identify good data is crucial for success,” he adds. When using scleral topography in clinical practice, it is important that ODs take into account that they may not be able to take images on the same day as the comprehensive exam, according to Dr. Sicks. “We typically recommend the patient be out of their habitual lenses for 48 hours before scans for best results. Thus, the patient often returns for an additional visit for scans.”
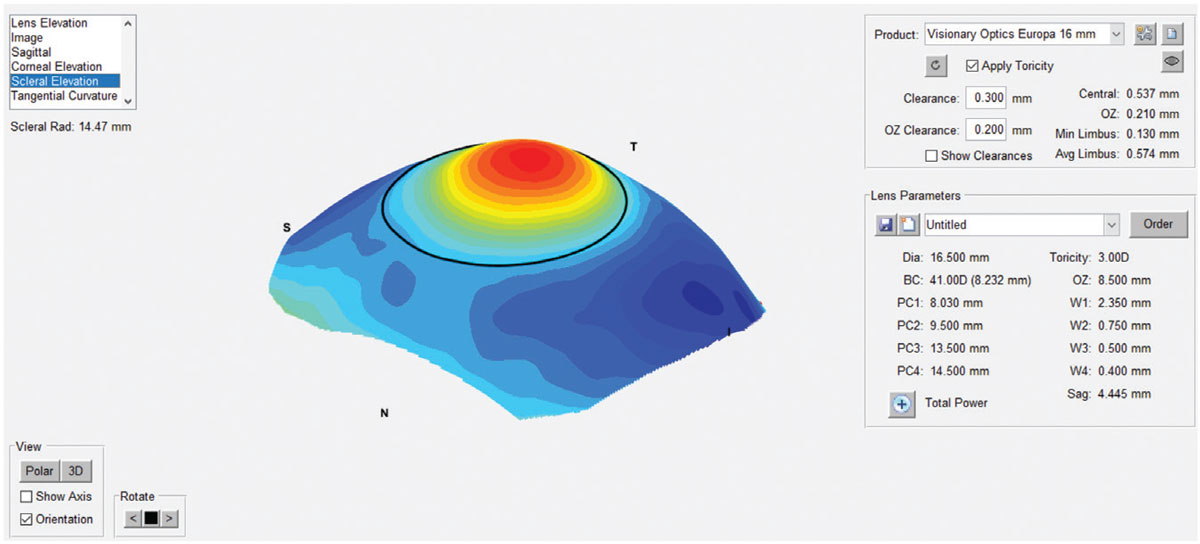 |
|
The sMap 3D scleral elevation map after image-stitching. Photo: Marcus Noyes, OD. Click image to enlarge. |
When determining if this device is the right fit, ODs must consider the unique needs and dynamics of their clinical practice. “While I believe that every patient should have the best fitting experience and care possible, this device may not make dollars and sense for every optometrist,” acknowledges Dr. Gelles. “That being said, scleral profilometry is a valuable addition and, if it is an option, I would encourage ODs to consider investing in this device for the benefit of their patients and practice.”
LLLT, IPL and Thermal Pulsation
Dry eye is an area of optometric care that’s teeming with new technology options that can improve outcomes. Tried-and-true medical therapies will always be a mainstay, but there are now several device categories that offer in-office procedures to accelerate symptomatic improvement.
There is a strong case, according to Paul Karpecki, OD, medical director of the Dry Eye Institutes of Kentucky and Indiana, for the use of low-level light therapy (LLLT), a photobiomodulation intervention, in the management of ocular surface disease. It can work as a standalone treatment or in conjunction with intense pulsed-light therapy (IPL).
“LLLT can come in various forms—ranging from blue, which has antimicrobial properties and can be used for blepharitis, including Demodex—to yellow for postsurgical edema to red for meibomian gland dysfunction (MGD), hordeola and chalazion,” explains Dr. Karpecki. Red LLLT is by far most commonly used.
In Dr. Karpecki’s experience, combining LLLT with IPL further enhances the effects of each when treating conditions such as MGD, evaporative dry eye or ocular rosacea. In the case of hordeola or chalazia, red LLLT alone is all that is needed, he notes. “In fact, I have only had to surgically remove two hordeola and inject two in the last two years since incorporating red LLLT alone for this condition.”
For Harriette Canellos, OD, associate clinical professor at SUNY College of Optometry, IPL is her go-to treatment for ocular rosacea. While she acknowledges that the initial cost of the device may be a factor, her clinic has found success with IPL. “The number of patients interested in the procedure will likely be greater than one might expect—especially when you educate them that IPL can also help treat fine lines and wrinkles.”
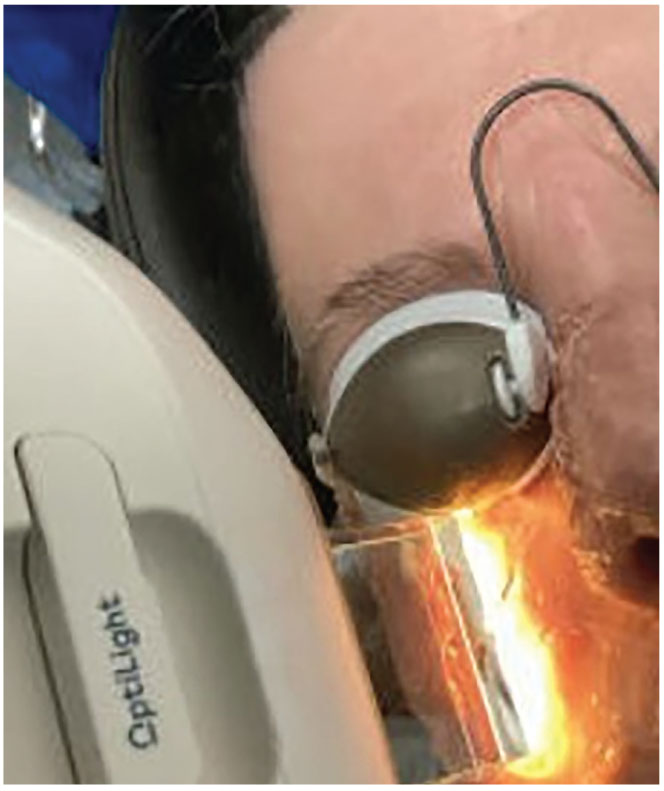 |
|
Intense pulsed-light therapy. Photo: Harriette Canellos, OD. Click image to enlarge. |
Thermal pulsation, which combines heat and gentle pulsating pressure, is primarily used to treat MGD. In addition to using the device to treat patients with MGD, Dr. Canellos also has many patients who report a reduction in their recurrent chalazia after LipiFlow (Johnson & Johnson). “It does not require much chair time,” she notes. “Once you place the applicators on, the procedure is automatic for 12 minutes and the doctor does not need to be present during that time.”
Dr. Canellos and her colleagues perform meibography on every patient. “We are able to show our patients the damage to their meibomian glands. Once they see these images, they start to understand the importance of taking care of their eyelid health and the value of thermal pulsation,” she says. The overwhelming majority of dry eye patients have MGD and Dr. Canellos believes that having a thermal pulsation device available as a treatment option is essential.
Each of these devices offers benefits for optometric practice. “IPL and LipiFlow work differently and, in my opinion, it is not a choice between having one or the other in practice,” she advises. “Many patients benefit from both treatments and a combination often gives the best results.”
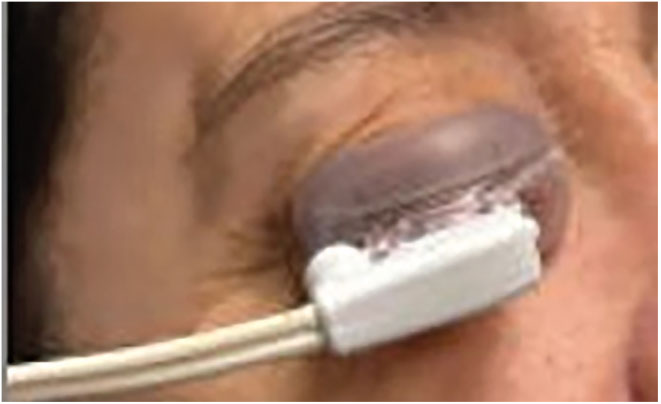 |
|
LipiFlow treatment. Photo: Harriette Canellos, OD. Click image to enlarge. |
In Dr. Canellos’ practice, a significant amount of time during initial consult is dedicated to discussing the patient’s diagnosis and all treatment options that pertain to the individual. “I do not promote one particular treatment but I recommend the ones I think will work best for each individual patient,” she notes. “It is important to me that my patients trust my care and treatment recommendations. I never want to give them the impression I am pushing a particular procedure for my financial gain.”
Tear Osmolarity
Dry eye is so familiar to optometrists that many can diagnose it literally with their eyes closed—just by talking to the patient about their symptoms and lifestyle. A simple slit lamp exam can then confirm it. However, some clinicians swear by the benefits of sophisticated measurements of tear film properties for both diagnostic purposes and long-term monitoring of treatment response.
Osmolarity testing is a measure of the level of solutes in a fluid, one that Dr. Karpecki considers an essential tool for any practice that treats ocular surface disease and wants to perform at the highest level. A reading above 308mOsm/L indicates dry eye disease and poor-quality tears. A difference of more than 6mOsm/L between eyes suggests tear instability, and the higher of the two eyes is the measurement to determine dry eye, he explains.
“The value of osmolarity testing lies in the area of efficiency. Not only does a reading only take about 12 to 15 seconds, but the efficiency in clinic is observed in making an accurate diagnosis of dry eye, determining a differential diagnosis and for patient education,” says Dr. Karpecki. “In my experience, it is the most reliable and accurate test I have.”
Toward the end of treatment, patients with longstanding dry eye often experience symptom relief, so showing continuous improvement in osmolarity allows them to know the treatment is helping and they are on track. Furthermore, patients are used to knowing their numbers, like when their blood pressure is checked or when glaucoma patients receive intraocular pressure measurements, according to Dr. Karpecki, and this is a similar scenario.
“If I don’t have access to osmolarity testing in my clinic, it’s difficult to know the diagnosis, difficult to teach patients or track progression and the clinic day is often running hours behind as we try to use less efficient tests to figure out the disease and if treatment is working,” Dr. Karpecki explains.
Dr. Canellos also considers this a key component of clinical practice. “We use TearLab (Trukera/Bausch + Lomb) as well as InflammaDry (Quidel) as part of our full dry eye work-up. TearLab evaluates tear osmolarity of each eye and InflammaDry tests for matrix metalloproteinase-9, which is an inflammatory marker elevated in tears of patients with dry eye,” she explains. Both are easy use, low-cost and billable to insurance, with the two also helping determine appropriate treatment strategies.
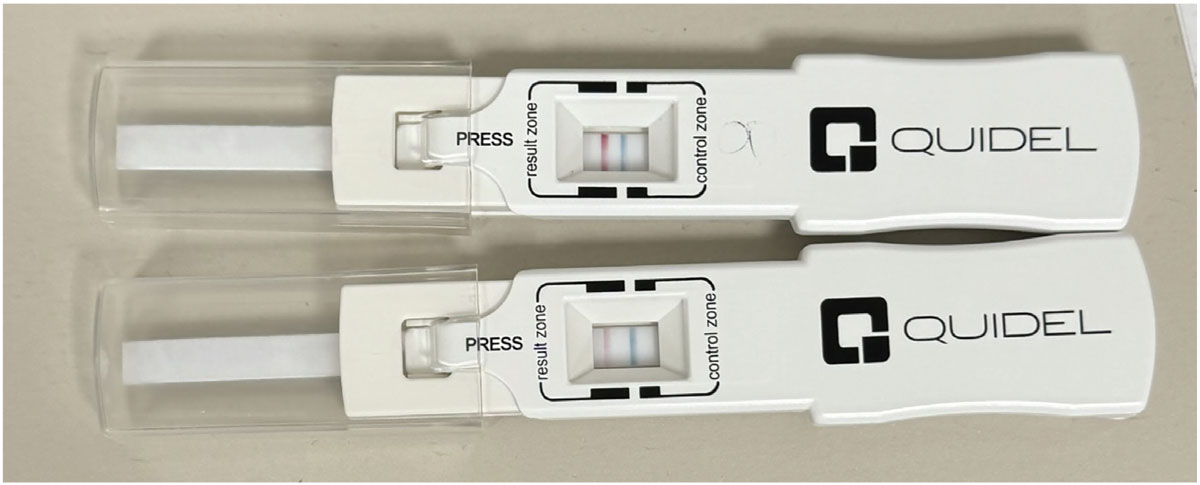 |
|
InflammaDry positive results for matrix metalloproteinase-9, an inflammatory marker elevated in tears of dry eye patients. Photo: Harriette Canellos, OD. Click image to enlarge. |
Wavefront Aberrometry
Another advanced diagnostic device that provide detailed information about optics of the eye to enhance specialty lens fitting, wavefront aberrometers also have use outside of the contact lens clinic, explains Dr. Gelles. They can aid in the understanding of patient visual complaints and can raise suspicion of disease affecting the optics of the eye and the need for further testing, according to Dr. Gelles. “By replacing an autorefractor with a wavefront aberrometer, much further insight is gained and can help a practitioner emphasize with a patient,” he offers.
By mapping aberrations with high precision, wavefront aberrometry enables optometrists to understand the visual quality of patients. With specific aberrometers, this data can be used to tailor the optics of scleral lenses to an individual’s unique visual needs. These wavefront-guided scleral lenses can provide significant improvements for those with complaints of poor visual quality in standard scleral lens optics.
The ability to customize lenses based on wavefront data helps improve visual clarity, reduce visual distortions and enhance overall visual performance, ultimately contributing to a more personalized and effective approach to vision correction, according to Dr. Gelles, who has found that the vast majority of patients prefer wavefront-guided lenses.
 |
|
A point spread function, shown here, can be calculated using wavefront aberrometry, which simulates how a patient would see a perfect white point on a black background; the left and right images depict two different patients. On the left is a normal cornea and on the right is an individual with keratoconus. Photo: John D. Gelles, OD, and Travis M. Pfeifer, OD. Click image to enlarge. |
“Patients want these optics,” he emphasizes. “They definitely offer an improved level of visual acuity, which provides patients with better functionality and overall quality of life. He says many patients are able to achieve an ‘age-matched normal’ level of vision quality with wavefront guided lenses. He notes that there is a strong case for the use of wavefront aberrometry in optometric practice, urging optometrists to consider if this is a device that would benefit their patients.
Dark Adaptometry
This tool has been shown to be a sensitive test for detecting early functional changes among patients with age-related macular degeneration (AMD). Studies have demonstrated that impaired dark adaptation (DA) often precedes visible structural changes, making it a potentially valuable tool for early diagnosis and intervention.
While a number of diagnostic tests for AMD exist, optometrists who concentrate on retinal disease can stay ahead of this condition by identifying AMD risk during routine examinations and using dark adaptation, which can identify the earliest functional biomarker of AMD, explains Amanda Legge, OD, practicing at the Wyomissing Optometric Center based in the Reading, PA area.1
“Most of the time with especially early AMD, there is not a definitive line that’s crossed when someone is diagnosed” with conventional methods, Dr. Legge says. However, she argues, “having any drusen with delayed dark adaptation = AMD.” She explains that the test is 90% specific, sensitive and accurate in differentiating small or pinpoint drusen from normal age-related changes vs. early AMD. It also helps identify patients with subretinal drusenoid deposits (SDDs). “These studies reveal that the presence of reticular pseudodrusen (RPD) is associated with an additional two- to sixfold increased risk of progression to neovascular AMD or central GA, with the risk even higher for RPD located outside the macula.”
SDDs appear in eyes that are clinically unremarkable, Dr. Legge explains, but their dark adaptation is deeply impaired even early on. “These are maculae that look clinically benign but have a dark adaptation of >20 minutes. When you know what and where to look on OCT to diagnose SDDs, this deep delay in DA usually signifies overall advanced AMD that matches the clinical picture OR the presence of SDDs even in a mild clinical appearance.”
Getting out in front of these disease processes can make a meaningful impact on the long-term course. “These patients can convert to choroidal neovascular membrane but were never told they even had AMD previously because of the mild clinical appearance,” she notes.
The AdaptDx (LumiThera Diagnostics) headset device measures a patient’s rod intercept (RI) time. This measurement is the number of minutes it takes for the eye to adapt from bright light to darkness. An RI time of less than 6.5 minutes suggests normal dark adaptation, indicating healthy photoreceptor function, while anything higher is a sign of impaired function, which is typically associated with AMD in patients over 50 years old.2
Dark adaptation serial testing over time also provides a functional measure of the patient’s AMD, Dr. Legge explains. “Those progressing quicker functionally (more delayed in DA with each test) are monitored more closely in office regardless of clinical appearance. It is a way to individualize treatment of AMD because every person is very different in AMD risk and management.”
Dark adaptometry has the potential to enhance retinal care in optometric practice. “It’s easily reimbursable and we have very few (if any) denials for testing for drusen, AMD or acquired night blindness,” says Dr. Legge. It’s also convenient for the practice, as the test can be conducted in any room. However, it does take time—up to 20 minutes to complete both eyes, she says, adding that most do not take that long.
As with any technology, implementation requires careful consideration to determine whether it is the right fit for the needs of a specific clinical practice. If you don’t see a lot of elderly patients and emphasize AMD in your practice, it may not be a good fit.
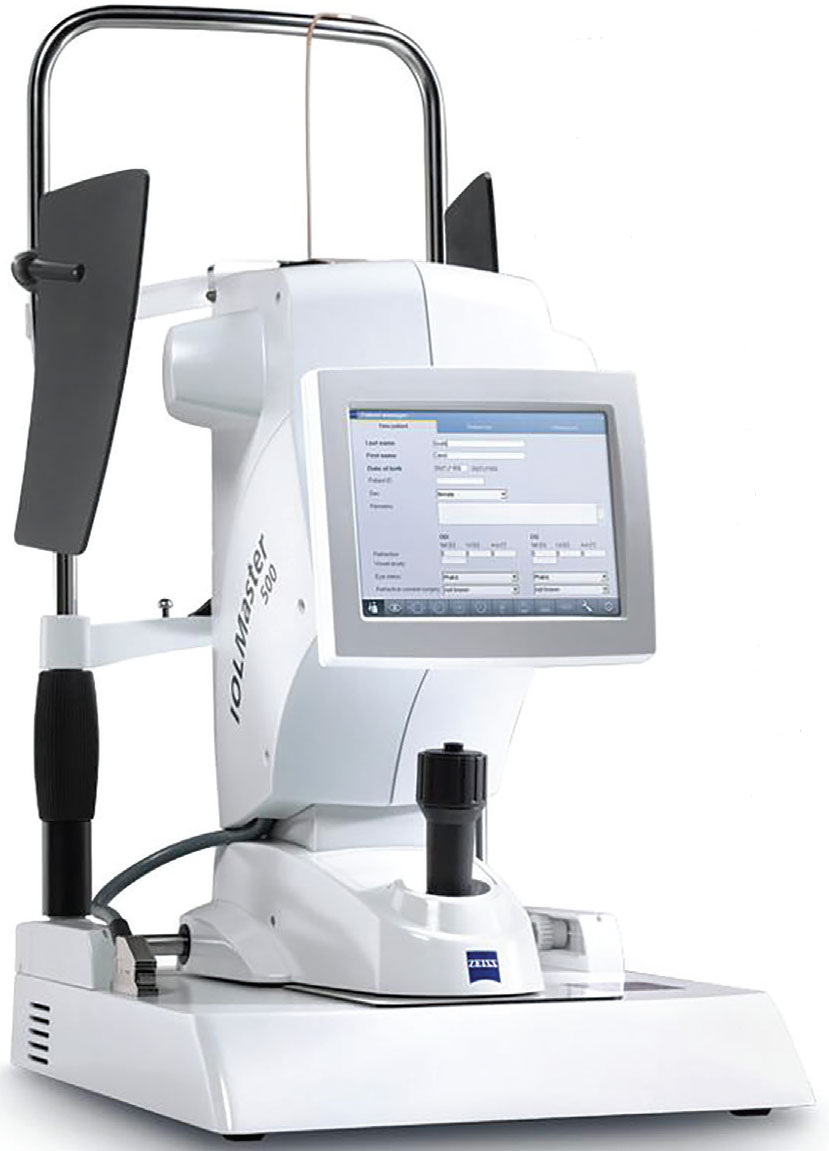
Optical Biometry
After scleral lenses, the other rising star in optometric care lately is myopia management. An optical biometer is key for those interested in adding this service, notes Dr. Sicks. The main goal with myopia management is to reduce axial elongation rate. “The only direct way to measure that rate is via optical biometry (a reduction in refractive progression does not always correlate with a reduction in axial length progression),” she says.
She adds that “it helps you monitor the success of your intervention,” while noting that, in her practice, she prefers to obtain biometry at baseline and then every six months thereafter.
Most instruments marketed for myopia management are non-contact, interferometry-based devices, which are more accurate (resolution to 0.03D) than older A-scan applanation ultrasound devices (resolution to 0.30D), according to Dr. Sicks. “Thus, I would recommend the interferometry-based devices to manage myopia; a non-contact instrument is preferable for pediatric patients as well. Some instruments also have additional risk factor analysis reports available.”
For Langis Michaud, OD, a professor of the School of Optometry at the L’École d’optométrie de Université de Montréal, biometry is the most important tool he employs in his clinic for the care of myopic children. “From the beginning, axial length—not refraction—along with growth charts, allows me to determine the risk of high myopia in adulthood,” he explains. “The higher the risk, the more aggressive my control management will be,” he explains.
 |
|
Optical biometry is an essential tool for any practice offering myopia management. Buying a used older model from a cataract surgeon can be an affordable option. Click image to enlarge. |
Dr. Michaud provides the example that an eight-year-old myopic child with -1.00D (cyclo ref) and an axial length of 23mm would not be treated the same as if 24.5mm was observed at baseline. “During follow-ups, axial length is the only parameter on which I base my assessment of the patient’s progression, especially for those fitted with orthokeratology lenses.”
While many might say that biometry is nice to have, but not a necessary tool for myopia management, Dr. Michaud considers it mandatory and standard of care: “Compare it to glaucoma. Can you consider managing that condition based solely on Goldmann intraocular pressure? The obvious answer is no.” To properly manage glaucoma, a practitioner needs optic nerve scans, visual fields, gonioscopy, etc. to make a proper assessment, he explains. Managing myopia without axial length measurements taken at baseline and follow-ups is risky, as is treating glaucoma based on intraocular pressure alone, Dr. Michaud comments. You can do it, but you can also miss the mark that way.
Investing in new equipment can often come with a hefty price tag. “Some of my colleagues argue that they cannot afford a biometer for their practice. Fortunately, there are many affordable options available today,” notes Dr. Michaud. “And we need to learn how to charge for our services. If you charge every time you use your biometer, it becomes a profit center, not an expense. More importantly, you will bring your practice up to the standard that our young patients and their parents/caregivers expect,” he adds.
Fundus Autofluorescence (FAF)
This type of retinal imaging is a useful tool for conditions that affect the outer retina— particularly the retinal pigment epithelium—and it is often incorporated into current imaging devices, such as OCT and fundus photography systems, Jessica Haynes, OD, of the Charles Retina Institute in Germantown, TN explains.
FAF can support the detection of geographic atrophy (GA) in AMD as well as monitoring progression of the disease. It can also help identify and diagnosis less common conditions, such as retinal diseases, macular telangiectasia type 2 and pattern dystrophies, among others, Dr. Haynes elaborates.
“Many imaging devices used by optometrists are already equipped with FAF capability or are modifiable to have FAF technology,” notes Mo Rafieetary, OD, another practicing physician at the Charles Retina Institute. When purchasing a new camera or OCT, optometrists may want to ask if it comes with FAF.
Presently, FAF is billed the same as fundus photography (92250) and does not have its own CPT code, explains Dr. Haynes. “This does limit its reimbursement, as FAF is often best interpreted alongside information from other retinal imaging that may not be billable on the same day, such as OCT and fundus photography.”
When deciding if FAF is a good fit for their clinical practice, ODs should take into consideration various factors, recommends Dr. Haynes. How many patients with retina disease are seen in your practice—particularly those with AMD or others that would benefit from FAF? Would having FAF allow you to better manage these patients or keep them in your practice longer?
“With new treatment options available to slow down the progression of GA secondary to AMD, it is critical to identify these patients and provide them with appropriate education and referral, when necessary,” advises Dr. Haynes. “FAF is a helpful tool in making this possible.”
Dr. Rafieetary adds that FAF also holds prognostic value. For instance, a hyper-AF border of a GA lesion suggests its progressive nature, he notes. “Once the eyecare provider becomes familiar with this imaging technique, they can explore its strengths and weaknesses, then the technique when indicated, can be used as another tool for better patient care.”
 |
|
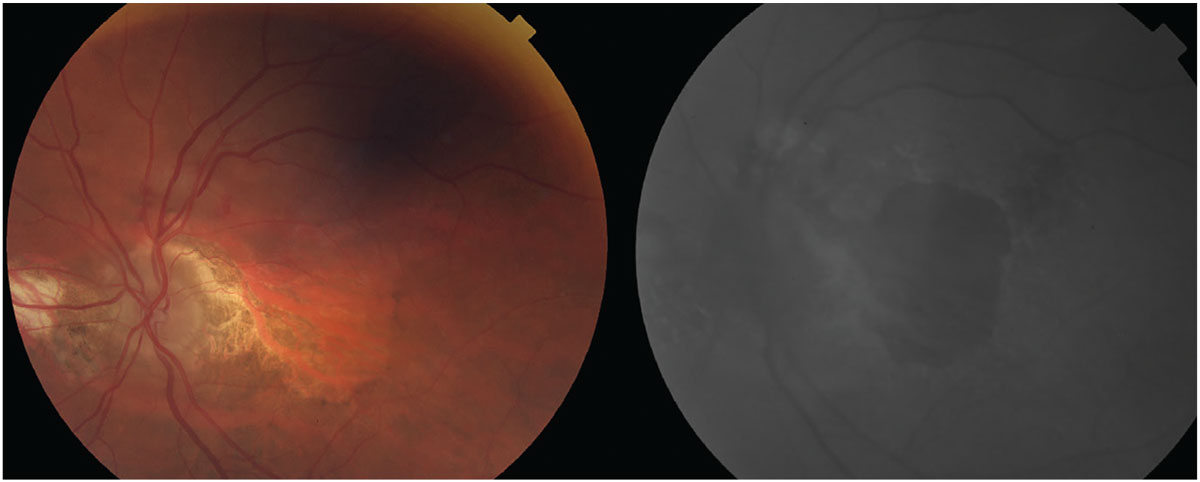
The left photo depicts advanced AMD with extensive, central GA. At right is an FAF photo showing extensive amounts of central hypofluorescence due to retinal pigment epithelium loss and a small area of hypofluorescence superior to the optic nerve caused by increased metabolic activity from the neovascular membrane. Photo: Steve Njeru, OD, MS, and Daniel Grangaard, OD. Click image to enlarge. |
Electroretinogram (ERG) Testing
This type of device can give early and objective indicators in diagnosis and management of ocular diseases affecting the retina, particularly diabetic retinopathy (DR), retinitis pigmentosa and AMD.
“I think of ERG as analogous to an electrocardiogram of the retina,” notes Paul Chous, OD, who has a private practice specializing in diabetes eye care in Tacoma, WA. “It essentially measures the electrical current generated by various retinal cell subtypes—photoreceptors, bipolar cells, ganglion cells—to varying intensities of a flickering light stimulus.”
Discussing clinical applications ERG, Dr. Chous says, “We all know that 100% contrast visual acuity is affected late in many retinal disorders, including DR. Full-field ERG allows clinicians to assess the function of the entire retina and then compare that function with structural examination findings such as those from dilated funduscopy, retinal photography, fluorescein angiography and both conventional OCT and OCT angiography.”
What is interesting is that some patients have relatively mild DR based on clinical exam but poor retinal function using full-field ERG, Dr. Chous observes, who notes that this structure-function discrepancy can help optometrists reassess clinical findings and even reconsider referral to retina specialty.
“In fact, abnormal full-field ERG results, in combination with diminished pupillary responses to the same stimulus, have been shown to predict which DR patients are most likely to require a retinal intervention (laser or intravitreal injection) over the next three years,” he explains. “Just like in glaucoma, we are trying to look for structure-function agreement or disagreement and more actively monitor and/or intervene in patients with DR.”
 |
|
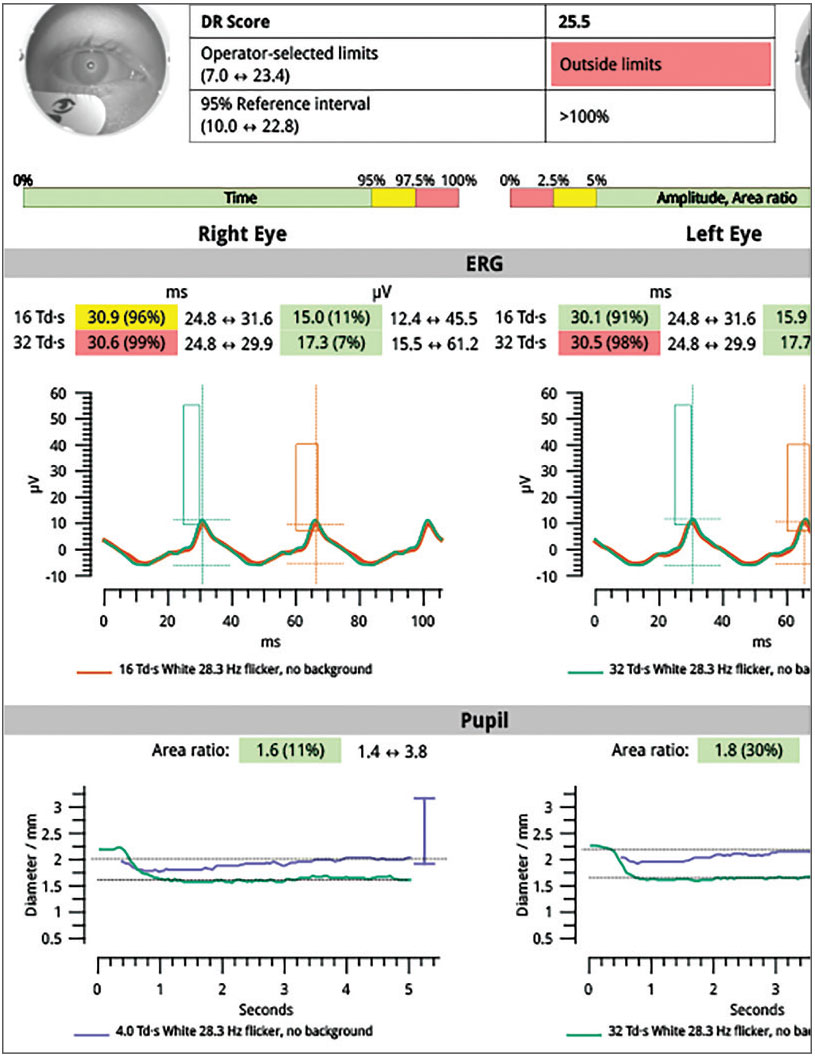
Full-field ERG wave form showing delayed signal (longer implicit time) and reduced amplitude. Diminished pupillary response differential between brighter and dimmer full-field ERG stimulus. Photo: A. Paul Chous, OD. Click image to enlarge. |
When integrating this technology into clinical practice, Dr. Chous recommends that ODs consider the number of patients they examine with diabetes and DR, the relatively easy use of full-field ERG technology in daily practice (about five minutes from start to finish), the objective nature of ERG vs. other subjective functional tests commonly performed (visual acuity and perimetry) and return on investment. Shortcomings can include ERG’s relative lack of reference database guidance compared to technologies like OCT and uncertainty on indications for use in various clinical scenarios.
“The most important question, in my view, is whether this technology benefits patients and, in my diabetes-centric practice, it certainly does,” he says. “My experience with ERG has been positive, and it’s been interesting to see some patients’ retinal function significantly improve with better metabolic control, anti-VEGF therapy or nutraceutical intervention.”
 |
|
The RetEval handheld ERG device. Photo: LKC Technologies. Click image to enlarge. |
Head-Mounted VF Testers
A more patient-friendly approach to perimetry, “virtual reality” visual field instruments represent a significant advancement in the convenience and patient-friendliness of such tests.
Humphrey visual field testing remains the gold standard, and while visual field instruments over the years have gotten somewhat smaller, there can still be space constraints, notes James Fanelli, OD, founder and director of Cape Fear Eye Institute in Wilmington, NC. He also notes that there can be challenges for certain patients, especially elderly individuals who cannot lean forward into these devices comfortably for the duration of the visual field tests.
After extensive consideration, Dr. Fanelli integrated a headset perimeter into his practice. “These instruments have transformed visual field testing in our office,” he says. “We no longer have the space constraints of a dedicated room for visual field testing. These portable, head-mounted devices can be used while a patient is waiting to be seen or during pre-testing. They can be moved around to different locations in the office, and, in fact, we have not used the ‘box’ perimeter in over a year and a half.”
While discussing concerns regarding reproducibility and reliability, Dr. Fanelli has found the virtual reality visual field tester he uses (VisuAll, Olleyes) is comparable to traditional devices. The one downside is that Humphrey information is not transferable, he notes. “However, you can compare two pages side by side (when you complete the first test) and pick up where you left off. You just don’t have the trend analysis converting from one to the other,” he cautions.
 |
|
Olleyes virtual reality visual field tester. Photo: Olleyes. Click image to enlarge. |
Unlike traditional perimeters, this type of device can test both eyes simultaneously, at once saving time and enhancing the patient experience, says Dr. Fanelli. Another benefit he outlines is the tracking capabilities available on these testers. “Fixation losses are always a problem with any type of visual field test, but what is nice about this device is if the patient does move their eye just before the stimulus is presented, the visual field unit will move the stimulus according to where their eye is, so fixation losses go down significantly and accuracy goes up,” he explains.
With a heavy glaucoma and neuro-optometric based practice, Dr. Fanelli and his partners are typically running these units all day. If an optometrist is in the market for a new visual field device, he highly recommends they consider the option of a virtual reality visual field tester, which allows ODs to perform comprehensive visual field testing with greater efficiency, accuracy and flexibility, elevating the overall quality of eye care.
Takeaways
Just as the many interviewees for this article focus on or specialize in certain areas of optometry, the devices they use and outline here also are geared toward certain specific uses. Not every optometrist will need every technology listed here, but certainly one may apply to your practice’s demands.
1. Legge A. How to stay one step ahead of AMD. Rev Optom. 2019;156(6):42-50. 2. Gerson J, Karpecki PM, Kirman G, et al. Practical perspectives on the diagnosis and management of AMD. Rev Optom. 2018;155(10):Suppl. |

