Due to last months U.S. Food and Drug Administrations approval of NearVision CK (Refractec), we are now able to offer our emmetropic and hyperopic presbyopic patients a low risk procedure that may not only enhance their lives, but may also offer them additional acuity.1 But before we discuss the procedure, we must first educate them about its roots, and remember that careful patient selection is essential.

CKs Roots
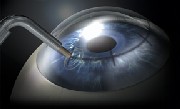
The FDA first approved CK, which stands for conductive keratoplasty, in April 2002 for the treatment of low to moderate hyperopia (+0.75D to +3.25D) in patients over 40. In CK, the surgeon inserts a thin probe that releases radio frequency energy in a circular pattern around the cornea (eight to 32 treatment points) to shrink small areas of collagen. This creates a constructive band that increases the corneas curvature.
Some Advantages to CK:
There is no tissue removal, which can lead to corneal ectasia or irregular flaps (striae, wrinkles or folds).
No treatments are done on the visual axis.
According to one study, some patients who undergo hyperopic CK may end up with a larger optical zone than those who undergo hyperopic LASIK and are less likely to experience starbursts and haloes.2
Other studies revealed mild hyperopic regression, (though it was low and decreased over two years postoperatively) and corneal iron ring deposits as CK risk factors.3-5 One case of corneal perforation was reported in a patient who underwent CK following previous refractive surgeries.6 This suggests surgeons need to take extra care that the CK-treated spots do not coincide with areas in the corneal stroma that might have been altered by previous refractive procedures. In addition, they need to make sure that peripheral pachymetry is performed, yielding a minimum of 560 microns.
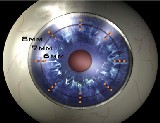
Above: CKs thin probe releases radio frequency in a circular pattern around the cornea. Right: The circular treatment pattern, printed on the cornea (in rinse away ink), is used to guide the doctors treatment. Courtesy of Refractec
The New Approval For CK
NearVision CK is indicated for the temporary improvement of near vision in emmetropic presbyopes (those who require only reading glasses) and hyperopic presbyopes (those who require reading and distance glasses). The procedure is typically performed on just one eye, improving near vision without compromising the patients binocular distance vision.
Refractec began FDA clinical trials using CK to treat presbyopia in October 2001 and concluded them in February 2003. The presbyopia trial consisted of 188 eyes (150 patients). The mean age was 53, the mean intended correction was +1.86D, and the corrections ranged from +0.75D to +3.00D. Some 77% of the subjects had an acuity of 20/20 at distance and J2 at near, and nearly 100% had acuity of 20/20 at distance and J5 at near.1
At six months post-operatively:
83% of those eyes treated for near had monocular uncorrected visual acuity of J3 at near.
81% of all patients had binocular UCVA of 20/25 or better at distance as well as J2 or better at 6Ma combination that represents for the presbyope, functional acuity. Only 9% of those treated eyes had this level of uncorrected binocular acuity preoperatively.
90% of all CK treated eyes had uncorrected binocular acuity of 20/32 at distance and J3 or better at 6M, which allowed for a high degree of uncorrected visual function.
At the 12-month follow-up, 97% of all the patients could see J5, while 87% could see 20/20 for the distance and read at J3 or better. Because there were no serious sight-threatening or unanticipated safety issues, the FDA approved NearVision CK on March 22, 2004.
Candidates
The ideal candidate for NearVision CK is past the age of 45, has less than 1.00D of refractive error and only requires reading glasses. CK is contraindicated in patients with chronic eye disorders, pregnant women, nursing mothers and patients with any chronic systemic illness or disease.7
Aside from educating the patient about the roots of CK and how the procedure works, it is also impertive to educate them about the healing process. Patients may experience tearing and some discomfort, including foreign-body sensation (which will occur in the first 24 to 48 hours) and itching.7
Also, instruct the patient to:
Not rub their eyes for two weeks following the procedure.7
Avoid any contaminated water sources including lakes, spas and swimming pools during the healing process.7
By educating ourselves and our emmetropic and hyperopic presbyopic patients about CKs roots, low risks and what can be expected post-operatively, this gives patients yet another exciting option to correct their visual needs.
1. Protocol RCS-011-PRS Conductive Keratoplasty US FDA Presbyopia Clinical Trial, January 22, 2003.
2. Rojas MC, Manche EE. Comparison of videokeratographic functional optical zones in conductive keratoplasty and laser in situ keratomileusis for hyperopia. J Refract Surg 2003 May-Jun;19(3):333-7.
3. Lin DY, Manche EE. Two-year results of conductive keratoplasty for the correction of low to moderate hyperopia. J Cataract Refract Surg 2003 Dec;29(12):2339-50.
4. Kymionis GD, Naoumidi TL, Aslanides IM, Pallikaris IG. Corneal iron ring after conductive keratoplasty. Am J Opthalmol 2003 Aug;136(2):378-9.
5. Probst LE, Almasswary MA, Bell J. Pseudo-Fleischer ring after hyperopic laser in situ keratomileusis. J Cataract Refract Surg 1999 Jun;25(6):868-70.
6. Kymionis GD, Titze P, Markomanolakis MM, et al. Corneal perforation after conductive keratoplasty with previous refractive surgery. J Cataract Refract Surg 2003 Dec;29(12):2452-4.
7. Frequently Asked Questions about Conductive Keratoplasty, Refractec website http://www.refractec.com/US/Patient/2140.asp.

