Papilledema is a condition that presents with bilateral optic nerve head edema due to increased intracranial pressure (ICP). This condition can be life-threatening and thus a medical emergency, so having a plan set in mind will help the optometrist.
History Considerations
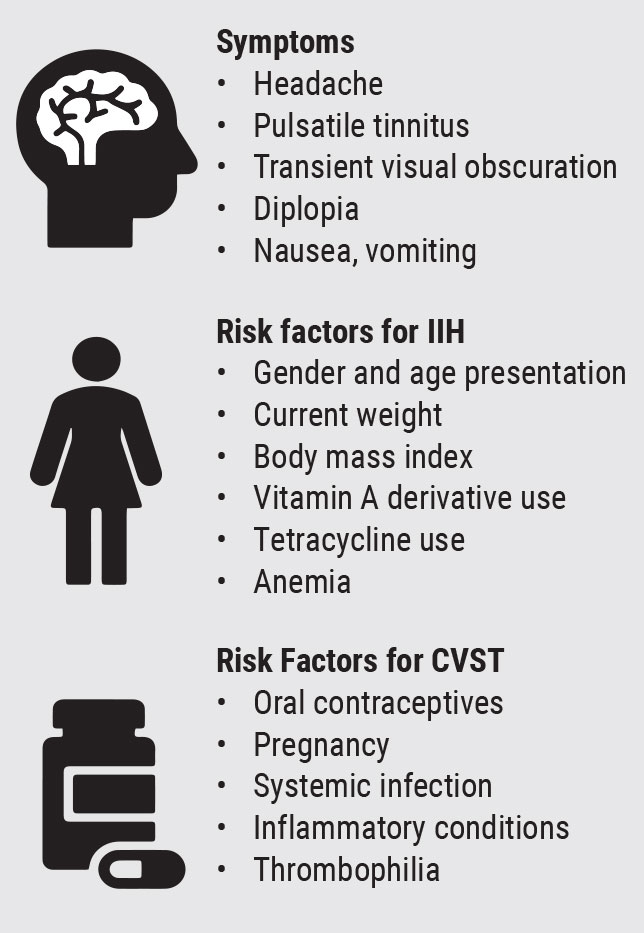
There are a number of pertinent case history questions that the clinician should ask in cases of suspected papilledema (Figure 1). First, ask about common symptoms and signs of increased intracranial pressure including headaches, transient visual obscurations, pulsatile tinnitus, nausea, vomiting and diplopia.1 Headaches are an especially common symptom associated with papilledema. The pain is often described as diffuse, may radiate down the posterior portion of the neck, and is characteristically more severe upon wakening in the morning and when laying down.
Transient visual obscurations are typically graying or blackening of the vision that only lasts a few seconds and are more common with a change in position.1 Therefore, the clinician should ask the patient, “Do you notice a change in your vision when you bend over to pick things up or get up from laying down?”2
 |
| Fig. 1. Pertinent symptom and history questions to consider for papilledema. Click image to enlarge. |
When asking about pulsatile tinnitus, describe the phenomenon as a whooshing sound with a rhythmic beat.3 It is important to differentiate this complaint from ringing in the ears (tinnitus) that can occur in conditions not related to increased ICP, such as hearing loss and Meniere’s disease. In contrast, pulsatile tinnitus is a sound synchronous to the patient’s pulse due to abnormal blood flow from increased ICP.
Papilledema can be secondary to numerous etiologies including, but not limited to, intracranial mass, venous sinus thrombosis and idiopathic intracranial hypertension (IIH).1 There are some questions that should be asked to assess for factors that place a patient at more risk for such etiologies.
If a patient’s papilledema is secondary to intracranial mass lesion, asking about neurologic symptoms, such as weakness and loss of sensation, may provide localizing details. Also consider inquiring about possible cranial nerve deficits, such as dysphagia and dysphonia, as these could point towards a brainstem lesion. Lastly, symptoms related to balance or gait issue could suggest cerebellar pathology to the optometrist.
Cerebral venous sinus thrombosis (CVST) occurs when a blood clot obstructs the cerebral venous drainage system, which in turn can then increase ICP.4 Signs and symptoms in patients with CVST depend on the location of the thrombus and resulting axonal injury and/or increased ICP. Therefore, presentations are variable ranging from mild headache to nausea, vomiting, focal or even diffuse neurologic deficits. Ask your patient history questions to assess for risk factors of CVST.5 These include underlying blood clotting disorders, such as sickle cell and thrombophilia, use of certain medications such as oral contraceptives, and infectious diseases.4
While rare, given the ongoing pandemic, clinicians may also consider the association between coronavirus disease 2019 (COVID-19) and underlying thrombosis in papilledema patients with this infection.-6 Other conditions that predispose patients to a hypercoagulable state include pregnancy, cancer and inflammatory conditions, such as lupus.
IIH commonly presents in females of childbearing age that are 10% or more above their ideal body weight. In these patients, neuroimaging must rule out structural etiologies such as mass or hydrocephalus. However, on neuroimaging, there are signs that can indicate increased ICP in the absence of structural lesions. These can include an empty sella, flattened posterior globe and dilated and tortuous subarachnoid space around the optic nerves.
Additionally, patients with IIH should have a normal cerebrospinal fluid analysis and an increased opening pressure on lumbar puncture.1 Since weight is a modifiable factor in IIH, it is important to ask about the patient’s current weight and monitor for changes at follow-up exams. Clinicians should also inquire about anemic states and the use of vitamin A derivatives and tetracyclines as these may be associated with IIH.7-9
Afferent Examination
A full assessment of afferent function is vital in any patient with papilledema. Clinicians must establish baseline visual function as treatment protocol may differ in cases with vision loss versus cases without. A baseline assessment may also contribute to determining effectiveness of treatment and monitoring for long-term damage.
A list of afferent testing to consider are seen in Figure 2. Patients will often present with relatively normal afferent function and the absence of afferent abnormalities should not exclude the diagnosis of papilledema. However, if assessed carefully, one might note subtle afferent findings, such as an enlarged blind spot which can be present even in early cases of papilledema.
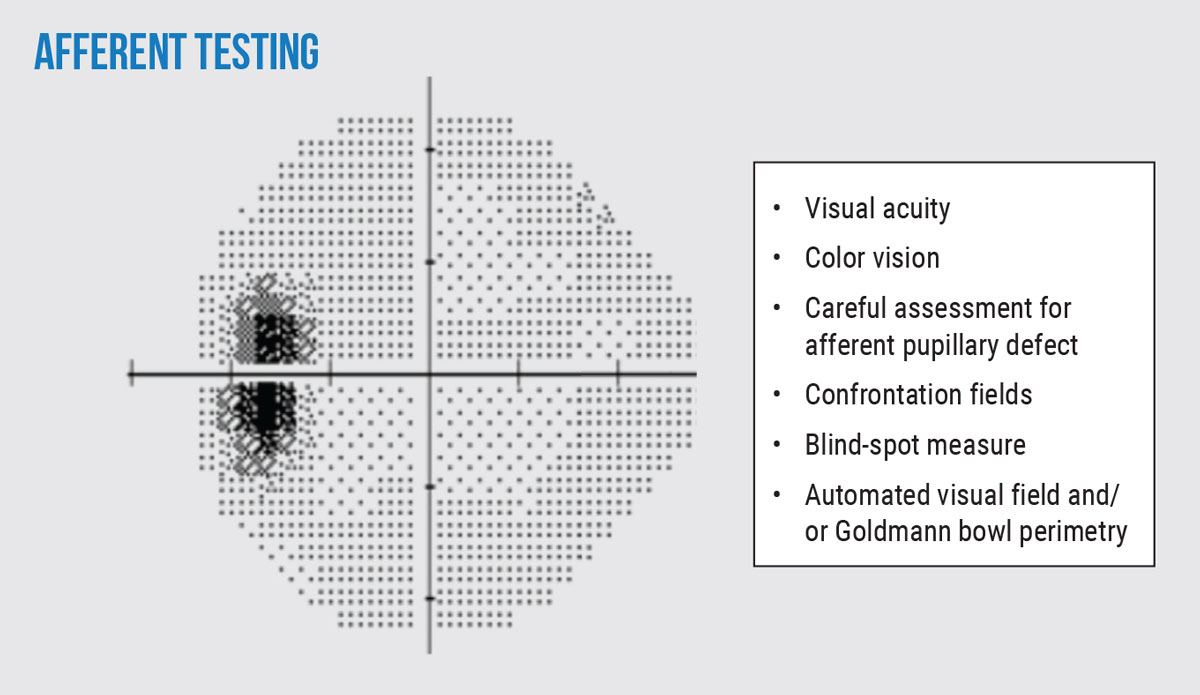 |
| Fig. 2. Pertinent afferent tests to perform in patients with suspected or confirmed papilledema. Consider these tests during both baseline and follow-up examinations for comprehensive monitoring. Note the enlarged blind spot on automated 24-2 visual field seen in the left eye of a patient. Click image to enlarge. |
One can consider assessing blind spot on confrontation fields by comparing the size of your own (so long as it is normal) to the size to the patient’s. The blind spot size averages 5.5º horizontal and 7.5º vertical.10 In cases of chronic papilledema, abnormal afferent functions may further manifest and are extremely important to identify as treatment may need to be modified. The long-term pressure on the optic nerves causes retinal nerve fiber layer (RNFL) damage resulting in findings such as reduced visual acuity, color vision and visual field defects.11
Efferent Assessment
Providers will want to note if there is any efferent abnormality, such as a cranial nerve (CN) palsy III, IV and/or VI palsy, in patients with papilledema as this could help to localize a potential space occupying lesion. CN VI is particularly susceptible to increased ICP due to its path through Durello’s canal. Thus, even patients without brainstem lesions may present with this palsy when increased ICP leads to compression of the nerve in this region. It is important to note that in addition to the optic nerve dysfunction, CN VI palsies are the only other acceptable abnormality on the neurologic examination in patients with the diagnosis of IIH.1,4
A combination of ductions and cover tests in multiple positions of gaze can help identify even the mildest of deficits. While performing ductions, be sure to ask the patient to extend their gaze as far as possible. The examiner should shift their own viewpoint to be sure to assess for any evidence of scleral show carefully (Figure 3).
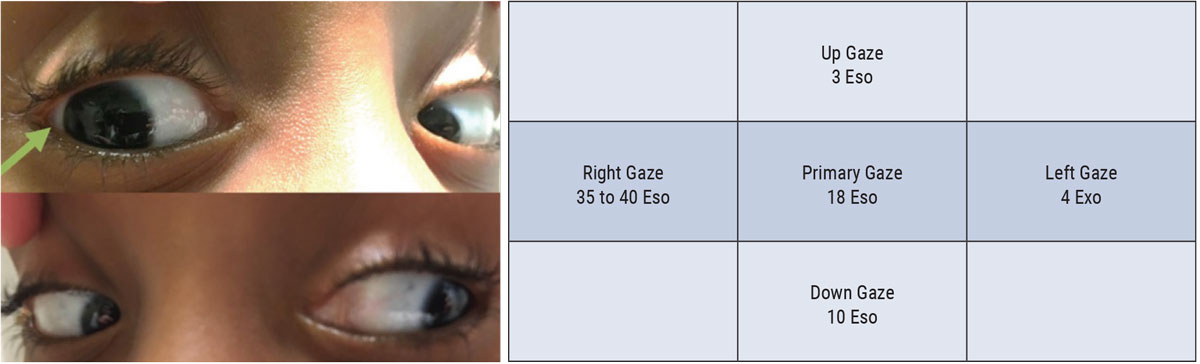 |
| Fig. 3. This patient from this efferent assessment presented with diplopia and was diagnosed with a CN VI palsy. Careful ductions demonstrated a subtle right abduction deficit. Cover testing demonstrated an increasing eso-deviation in right gaze, consistent with the patient’s right CN IV palsy. Click image to enlarge. |
Cover testing in multiple positions of gaze can be performed at distance to assess the patient for comitancy. Perform this testing without the patient’s glasses as they can block patients eccentric viewing and/or produce prismatic effect that can compromise results. Non-comitant deviations on cover testing can suggest a CN palsy. For instance, an increasing eso-deviation on lateral gaze can suggest a CN VI palsy ipsilateral to the direction of increasing misalignment.
Funduscopic Assessment
Papilledema has certain fundoscopic characteristics that should be carefully assessed for. A stepwise approach to assessing the optic nerve head on dilated exam will help determine if the patient has disc edema. First, assess each quadrant of the optic disc for any elevation. Next, assess the margins of the optic disc, evaluate for any margins that are blurry or indistinct. Further assess the margins for vessel obscuration; look at the small vessels at the edge of the margin and determine if there are segments missing of the vessel.12
Staying close to the margin of the optic nerve head on the temporal side, look for Paton’s lines, which are concentric folds of the retina.13 After full assessment of the optic nerve head, the fundoscopic findings can be graded from 0 to 5 in relation to the Frisen scale.14
The presence or absence of spontaneous venous pulsations (SVPs) is an important finding when assessing patients with possible papilledema. SVPs are caused by variations in the pressure gradient along the retinal vein as it emerges through the lamina cribrosa. It has been found that when a patient’s cerebral spinal fluid (CSF) pressure is higher than 190mm H2O, the CSF pulse pressure rises to equal the intraocular pressure causing the SVP to cease.15 Therefore, the presence of an SVP does suggest normalized ICP. However, approximately 10% of the normal population does not exhibit a physiologic SVP.
Thus the absence of an SVP should not be interpreted as definitive increased ICP. SVPs may be subtle and limited to a small segment of one vein; therefore, the provider must carefully assess for its presence on dilated examination. SVP should follow the rhythmic movement of the cardiac cycle and, if questionable, a provider should not rely on SVP to confirm or deny a diagnosis of increased ICP.15
Ancillary Testing
The use of optical coherence tomography (OCT) imaging of the peripapillary retinal nerve fiber layer (RNFL) and ganglion cell complex (GCC) in patients with elevated neuro-retinal rim can be useful in a multitude of ways. While the diagnosis of papilledema is likely made from the funduscopic view in moderate to severe cases, OCT can be a useful baseline assessment for future monitoring. However, in severe cases of papilledema, the scan may not be able to penetrate to deeper values and thus results may be less accurate.16
When assessing the peripapillary RNFL of a child, in which the OCT does not have a normative database currently, a clinician should reference values in literature to help determine if their patient’s OCT results are abnormal. One study found that the mean peripapillary RNFL thickness in children ages five to 15 in North America was 107.6µm.17 However, the RNFL thickness value alone is unlikely to be sufficient enough to differentiate cases of mild papilledema from pseudo-papilledema.18 Fortunately, there are a number of other signs on OCT images that may help to support the clinician’s diagnosis.
It has been theorized that the force of increased subarachnoid pressure in patients with papilledema may result in an anterior displacement of structures in the peripapillary region. Specifically, Bruch’s membrane (BM) and the retinal pigmented epithelium (RPE) have been shown to have an increased angle toward the vitreous in these patients, while BM and RPE in patients with disc swelling unrelated to intracranial hypertension was angled away from the vitreous.19
Additionally, measurements of the inward displacement of Bruch’s membrane have been shown to be statistically significant in being able to differentiate mild papilledema from pseudo-papilledema.20
However, there is no current standardized algorithm for analysis of the angle in which BM/RPE are located. Clinicians can currently assess a patient’s peripapillary anatomy using cross-sectional OCT images, such as with a raster scan, and if there is definite protrusion of BM/RPE towards the vitreous, then the diagnosis of papilledema should be presumed. Until standardized algorithms are clinically available, the absence of deflection should not rule out papilledema.
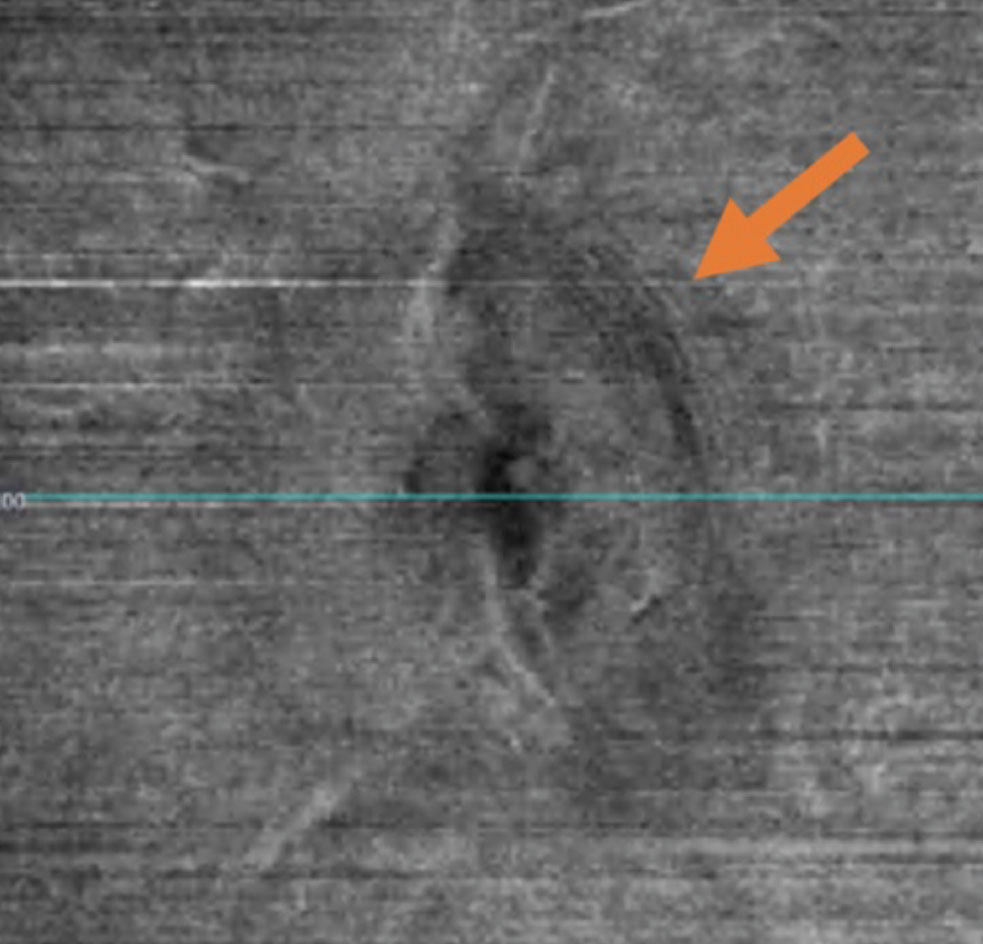 |
| Fig. 4. Peripapillary wrinkles seen on en face VRI OCT imaging. Click image to enlarge. |
The biomechanical forces of increased ICP give rise to concentric curvilinear folds of retina adjacent to the optic disc. Superficial folds of RNFL, known as peripapillary wrinkles or Paton’s lines, strongly suggest the diagnosis of papilledema and are often assessed with dilated funduscopic examination. OCT imaging of the peripapillary RNFL can be a useful adjunct in looking for this anatomical change.21 Specifically, en face vitreoretinal interface (VRI) OCT images may be able to highlight peripapillary wrinkles which are otherwise difficult to see funduscopically (Figure 4). OCT can also help to confirm the presence of papilledema in patients without peripapillary wrinkles in primary gaze. It has been demonstrated that placing the eye in the adducted state can elicit their presence and detection, including with OCT imaging.22
OCT may help to discern optic disc drusen (ODD) which, when buried, is a well-known mimicker of papilledema. ODD have been defined as signal poor lesions with overlying hyperreflective cap (Figure 5A).23 Peripapillary hyperreflective ovoid mass-like structures (PHOMS) have also been associated with ODD.24 However, clinicians must always be suspicious of papilledema overlying ODD, and it is important to note that PHOMS have now been identified in cases of papilledema and other pathologies.25 The presence of superficial ODD can also be highlighted on fundus autofluorescence (FAF) as bright concentric lesions (Figure 5B).
Other ancillary tests to consider on patients with ODD and/or papilledema include ultrasound of the optic nerve and fluorescein angiography (FA). In orbital ultrasonography, the optic nerve sheath width (ONSW) widens with increased ICP. Increased ICP also causes a change in the ONSW in primary gaze vs. upon 30º of abduction. Drusen will present as ovoid hyperreflective structures.26 With FA, drusen present as bright ovoid structures, staining in early and late stages and papilledema presents as leakage in the peripapillary region.
FA may be the modality of choice in pediatric patients, as their optic disc drusen are more likely to be buried, thus making detection on OCT and FAF more difficult.27
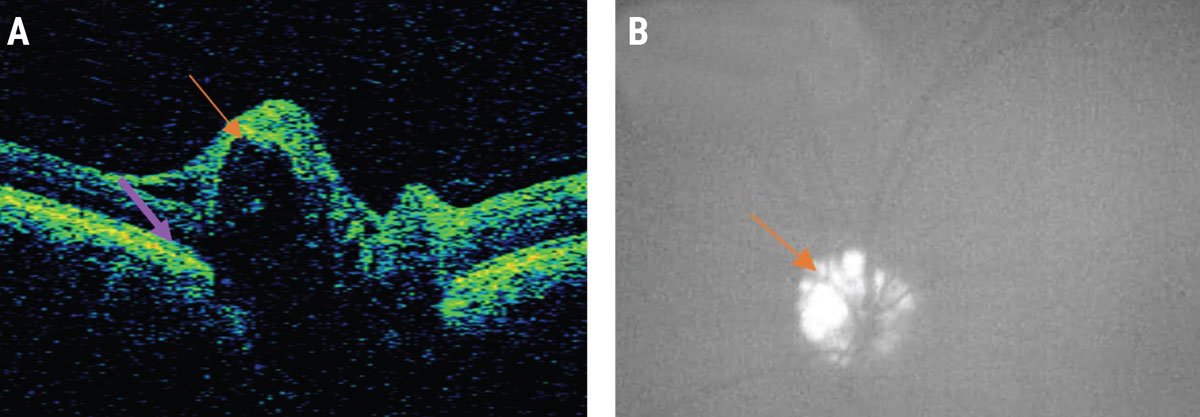 |
| Fig. 5. A patient with pseudo-papilledema: (A) Note the ODD (orange arrow) in this OCT image, characterized by a signal poor lesion with a hyper-reflective cap. Also seen is the downward deflection of BM/RPE in a patient without papilledema denoted by the purple arrow. (B) Superficial ODD in the same patient seen as bright lesions on fundus autofluorescence. Click image to enlarge. |
Management Strategies
If all of the signs are pointing to pseudo-papilledema without overlying papilledema, the clinician should consider asking the patient to return for close monitoring within one to two months. Stability of all findings, including afferent, efferent and ancillary tests, on subsequent examination may help to support the suspected diagnosis of pseudo-papilledema.
On the contrary, if there is suspicion of papilledema, the patient must be sent immediately to the hospital for further evaluation and neuroimaging, preferably magnetic resonance imaging (MRI) of the brain with and without contrast to rule out intracranial mass and magnetic resonance venogram (MRV) to assess for venous sinus thrombosis. Additionally, arterial imaging will help rule out arteriovenous malformations, especially in male patients in which no other etiology has been identified.28 If not contraindicated, lumbar puncture with opening pressure and analysis of contents, to rule out some etiologies such as infection, should then be considered.4 Ultimately, the differentiation between pseudo-papilledema and mild papilledema often remains a diagnostic challenge, but it is important that the clinician always considers that papilledema may have serious underlying etiologies, and additional evaluation and treatment must not be delayed.
Once a patient is definitively diagnosed with papilledema, our role as eye care providers does not end. As mentioned previously, OCT imaging can be helpful in the long-term monitoring of patients who have established care with neurology and begun treatment. Clinicians must always interpret subsequent OCT scans and their trends with caution.
While decreasing RNFL values may signify improving papilledema, this change must be differentiated from papilledema-related atrophy. Analysis of the ganglion cell complex is often a helpful discriminator, as thinning can be an early sign of papilledema related optic atrophy.29 The presence of GCC thinning may be associated with a visual field defect, and as more aggressive treatment is often warranted in patients with visual field defects and loss, providers must continually monitor for these changes.
In addition to GCC analysis, re-analysis of the BM/RPE angle may provide useful. It has been shown that the angle of BM/RPE changes promptly following lumbar puncture in patients with increased ICP.30 Patients with decreasing RNFL values secondary to papilledema related optic atrophy may still exhibit a positive BM/RPE angle towards the vitreous signifying that the patient still has active increased ICP.31
Takeaways
While it’s true that cases of papilledema may seem challenging, optometrists can increase their diagnostic confidence and improve patient outcomes with a thorough case history, careful examination and analysis of ancillary testing, such as OCT.
Dr. Maglione works in the neuro-ophthalmic disease services at The Eye Institute and teaches didactically in neuro-anatomy and neuro-ophthlamic disease courses at the Pennsylvania College of Optometry at Salus University. She is a Fellow of the American Academy of Optometry. Dr. Marunde is a neuro-ophthalmic disease resident at The Eye Institute at Salus University. They have no any financial interests to disclose.
1. Rigi M, Almarzouqi SJ, Morgan ML, Lee AG. Papilledema: epidemiology, etiology and clinical management. Eye Brain. 2015;7:47-57. 2. Sergott RC. Headaches associated with papilledema. Curr Pain Headache Rep. 2012;16(4):354-8. 3. Hofmann E, Behr R, Neumann-Haefelin T, Schwager K. Pulsatile tinnitus: imaging and differential diagnosis. Dtsch Arztebl Int. 2013;110(26):451-8. 4. O’Rourke TL, Slagle WS, Elkins M, et al. Papilloedema associated with dural venous sinus thrombosis. Clin Exp Optom. 2014;97(2):133-9. 5. Konakondla S, Schirmer CM, Li F, et al. New developments in the pathophysiology, workup and diagnosis of dural venous sinus thrombosis (DVST) and a systematic review of endovascular treatments. Aging Dis. 2017;8(2):136-48. 6. Ramesh SV, Ramesh PV, Ramesh MK, et al. COVID-19-associated papilledema secondary to cerebral venous thrombosis in a young patient. Indian J Ophthalmol. 2021;69(3):770-2. 7. Oldroyd CK, Walters M, Dani K. Raised intracranial pressure secondary to vitamin overdose. Am J Med. 2016;129(6):e9-10. 8. Kini AT, Rohani N, Othman BA, Lee AG. Recurrence of elevated intracranial pressure following tetracycline antibiotic use. Cutis. 2019;103(3):142-56. 9. Biousse V, Rucker JC, Vignal C, et al. Anemia and papilledema. Am J Ophthalmol. 2003;135(4):437-46. 10. Corbett JJ, Jacobson DM, Mauer RC, Thompson HS. Enlargement of the blind spot caused by papilledema. Am J Ophthalmol. 1988;105(3):261-5. 11. Schirmer CM, Hedges TR 3rd. Mechanisms of visual loss in papilledema. Neurosurg Focus. 2007;23(5):E5. 12. Yan Y, Liao YJ. Updates on ophthalmic imaging features of optic disc drusen, papilledema and optic disc edema. Curr Opin Neurol. 2021;34(1):108-15. 13. Sibony PA, Kupersmith MJ, Feldon SE, et al. Retinal and choroidal folds in papilledema. Invest Ophthalmol Vis Sci. 2015;56(10):5670-80. 14. Frisén L. Swelling of the optic nerve head: a staging scheme. J Neurol Neurosurg Psychiatry. 1982;45(1):13-8. 15. Jacks AS, Miller NR. Spontaneous retinal venous pulsation: aetiology and significance. J Neurol Neurosurg Psychiatry. 2003;74(1):7-9. 16. Malhotra K, Padungkiatsagul T, Moss HE. Optical coherence tomography use in idiopathic intracranial hypertension. Ann Eye Sci. 2020;5:7. 17. Yanni SE, Wang J, Cheng CS, et al. Normative reference ranges for the retinal nerve fiber layer, macula and retinal layer thicknesses in children. Am J Ophthalmol. 2013;155(2): 354-60.e1. 18. Vardanian Vartan C, Nguyen AM, Balmitgere T, et al. Detection of mild papilloedema using spectral domain optical coherence tomography. Br J Ophthalmol. 2012;96(3):375-9. 19. Kupersmith MJ, Sibony P, Mandel G, et al. Optical coherence tomography of the swollen optic nerve head: deformation of the peripapillary retinal pigment epithelium layer in papilledema. Invest Ophthalmol Vis Sci. 2011;52(9):6558-64. 20. Pardon LP, Cheng H, Tang RA, et al. Custom optical coherence tomography parameters for distinguishing papilledema from pseudopapilledema. Optom Vis Sci. 2019;96(8):599-608. 21. Sibony PA, Kupersmith MJ; OCT Substudy Group of the NORDIC Idiopathic Intracranial Hypertension Treatment Trial. Paton’s folds revisited: peripapillary wrinkles, folds and creases in papilledema. Ophthalmology. 2016;123(6):1397-9. 22. Sibony PA, Hou W. Adduction-induced deformations evoke peripapillary folds in papilledema. Ophthalmology. 2019;126(6):912-4. 23. Malmqvist L, Bursztyn L, Costello F, et al. The optic disc drusen studies consortium recommendations for diagnosis of optic disc drusen using optical coherence tomography. J Neuroophthalmol. 2018;38(3):299-307. 24. Mezad-Koursh D, Klein A, Rosenblatt A, et al. Peripapillary hyperreflective ovoid mass-like structures-a novel entity as frequent cause of pseudopapilloedema in children. Eye (Lond). 2021;35(4):1228-34. 25. Borrelli E, Barboni P, Battista M, et al. Peripapillary hyperreflective ovoid mass-like structures (PHOMS): OCTA may reveal new findings. Eye (Lond). 2021;35(2):528-31. 26. Neudorfer M, Ben-Haim MS, Leibovitch I, Kesler A. The efficacy of optic nerve ultrasonography for differentiating papilloedema from pseudopapilloedema in eyes with swollen optic discs. Acta Ophthalmol. 2013;91(4):376-80. 27. Chang MY, Velez FG, Demer JL, et al. Accuracy of diagnostic imaging modalities for classifying pediatric eyes as papilledema vs. pseudopapilledema. Ophthalmology. 2017;124(12):1839-48. 28. Schön S, Blackham K, Zumofen D, Mariani L. Papilledema in brain avms: pathophysiologic considerations on the basis of a case report. Neurographics. 2018;8:254-7. 29. Marzoli SB, Ciasca P, Curone M, et al. Quantitative analysis of optic nerve damage in idiopathic intracranial hypertension (IIH) at diagnosis. Neurol Sci. 2013;34 Suppl 1:S143-5. 30. Gampa A, Vangipuram G, Shirazi Z, Moss HE. Quantitative association between peripapillary Bruch’s membrane shape and intracranial pressure. Invest Ophthalmol Vis Sci. 2017;58(5):2739-45. 31. Sibony P, Kupersmith MJ, Honkanen R, et al. Effects of lowering cerebrospinal fluid pressure on the shape of the peripapillary retina in intracranial hypertension. Invest Ophthalmol Vis Sci. 2014;55(12):8223-31. |

