
|
Review of Systems for Graves’ Disease
|
The state of normal thyroid function is called euthyroidism, but abnormalities of the thyroid gland are fairly common, affecting 1% to 5% of the population, mostly women.1 Disorders of the thyroid gland cause hormone imbalance, which can complicate personal health in many ways.
The Axis
The thyroid gland is attached to the lower part of the larynx and upper part of the trachea. It has two sides (or “lobes”), each about 4cm long and 1cm to 2cm wide, which are connected by a narrow isthmus.2 The thyroid gland is influenced by the pituitary gland, which produces thyroid-stimulating hormone (TSH), and the hypothalamus, a small part of the brain above the pituitary, which produces thyrotropin-releasing hormone (TRH).2
The hypothalamus and pituitary detect low levels of thyroid hormones in the blood. When TRH is released, it stimulates the pituitary to release TSH. In turn, increased levels of TSH stimulate the thyroid gland to produce more thyroid hormone, thereby returning the level of thyroid hormone in the blood back to normal.2,3 The three structures and the hormones they produce make up the hypothalamic-pituitary-thyroid axis.
Graves’ and TED
Thyrotropin is a glycoprotein that stimulates the thyroid gland to produce and secrete the hormone thyroxine. Graves’ disease (GD) is an autoimmune disorder characterized by hyperthyroidism—the overproduction of thyroid hormones—due to circulating autoantibodies. Thyroid-stimulating immunoglobulins (TSIs) bind to and activate thyrotropin receptors, causing the gland to grow and its follicles to increase synthesis of thyroid hormone.4 While several conditions may result in hyperthyroidism, GD is the most common. Because thyroid hormones affect multiple body systems, signs and symptoms associated with GD are wide ranging.
Thyroid eye disease (TED), which has also been referred to as Graves’ orbitopathy or ophthalmopathy, affects up to 60% of patients with GD. Despite our detailed understanding of the etiology of hyperthyroidism in GD, the pathogenesis of TED remains uncertain.4 This has limited the development of targeted therapies, particularly those that alter the course of TED.
Signs of TED
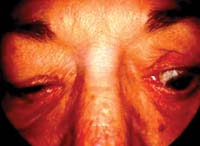 |
|
Evaluate the patient for upper eyelid abnormalities, such as retraction and von Graefe's sign.
|
In GD, the onset of hyperthyroidism and TED usually occur within 18 months of one another. A small minority of patients never develop thyroid dysfunction and are referred to as having “euthyroid GD.”4,5 The majority of patients with TED have mild, self-limiting disease. Nonetheless, even patients with mild disease experience a reduced quality of life.
Diagnostic Work-up
Diagnostic testing of free T4 (thyroxine) and TSH or serum TSH (thyrotropin) are highly sensitive and specific. Serum TSH is useful to establish a diagnosis of hyperthyroidism or hypothyroidism. Usually, the TSH is low in hyperthyroidism and high in hypothyroidism. Radiologic testing using a small amount of radioactive iodine may be implemented. A high uptake of radioactive iodine indicates that the thyroid gland is overproducing hormones.
Neuroimaging may be necessary if a diagnosis of TED cannot be established clinically. MRI is more sensitive than CT in showing compressive optic neuropathy. CT scanning usually reveals thick extraocular muscles with tendon sparing. The inferior rectus and medial rectus muscles are usually involved.
Treatment and Management
• Graves’ Disease. Treatment of GD centers on correction of the thyrotoxic state. Normalization of thyroid hormone levels can be achieved with agents that block the synthesis of thyroid hormones or by treatment with radioactive iodine. Systemic beta-adrenergic blockers are prescribed to reduce the effect of hormones on the body.4,5
• Mild/Moderate TED. Most patients with TED can be observed, with the follow-up interval depending on disease status. Evaluation for corneal exposure, optic neuropathy and diplopia should be performed at these visits. Visual field and color vision testing may help in early detection of visual loss.4,5
The use of prism may be beneficial to patients with diplopia, if the deviation is small-angle and relatively comitant. If large-angle and/or incomitant, tape occlusion of one lens or segment of the glasses may be helpful. If this doesn’t work, an occluder or vaulted eye patch (with care not to touch the cornea or compress the orbit) may be indicated. If a patient has dry eye symptoms, prescribe preservative-free artificial tears during the day and lubricating ointment at night, and consider punctal plugs.
• Severe TED. Systemic steroids represent the primary treatment for patients with moderate to severe, active TED. An IV steroid of 250mg of methylprednisolone given weekly over six weeks can be effective and have fewer side effects compared to oral steroids.8 Surgical decompression of the orbit may be considered when vision is threatened by compressive optic neuropathy. Orbital radiotherapy remains an adjunctive strategy. Targeted immunotherapies have the potential to alter disease progression, but further evidence is needed to establish safety and efficacy.5,7
Some of the immunosuppressive agents are azathioprine, cyclosporine and methotrexate. In severe cases of TED, treatment should be customized for each patient in close collaboration with the endocrinologist.8
Patient education
Patients living with TED should be advised that the condition usually runs a self-limited, but prolonged, course over one or more years, and no immediate cure is available. In addition, we must encourage patients to stop smoking, to decrease the risk of congestive orbitopathy. Sleeping with the head of the bed elevated may decrease morning lid edema.
TED likely involves both genetic and environmental factors. Until ongoing research establishes a target antigen, clinicians must focus on early signs and symptoms, along with timely treatment and management.
1. Furszyfer J, Kurland LT, McConahey WM, Elveback LR. Graves’ disease in Olmsted County, Minnesota, 1935 through 1967. Mayo Clin Proc. 1970 Sep;45(9):636-44.
2. Cummings CW, Frederickson JM, Harker LA, et al. Thyroid anatomy. In: Otolaryngology - Head and Neck Surgery. 3rd ed. St. Louis, Mo: Mosby;1998:2445-9.
3. Thyroid gland. In: Williams PL, Bannister LH, Berry MM, et al., eds. Gray’s Anatomy. 38th ed. New York: Churchill Livingstone;1995:1891-6.
4. Ing E, Abuhaleeqa K. Graves’ ophthalmopathy (thyroid-associated orbitopathy). Clin Surg Ophthalmol. 2007;25:386-92.
5. Bartalena L, Pinchera A, Marcocci C. Management of Graves’ ophthalmopathy: reality and perspectives. Endocr Rev. 2000 Apr;21(2):168-99.
6. Brix TH, Kyvik KO, Christensen K, Hegedus L. Evidence for a major role of heredity in Graves’ disease: a population-based study of two Danish twin cohorts. J Clin Endocrinol Metab. 2001 Feb;86(2):930-4.
7. Genovese BM, Noureldine SI, Gleeson EM, Tufano RP, Kandil E. What is the best definitive treatment for graves’ disease? A systematic review of the existing literature. Ann Surg Oncol. 2013 Feb;20(2):660-7.
8. Smith D, Cockerham KP, Douglas RS. Thyroid eye disease: Current and Emerging Therapies. EyeNet. 2011 Nov/Dec;31-3.
9. NJ Friedman, PK Kaiser. Orbit. In: Essentials in Ophthalmology. 1st ed. Philadelphia:Saunders Elsevier;2007:113 -24.

