 Since its introduction more than 20 years ago, posterior segment imaging with optical coherence tomography has become the standard of care for diagnosing and managing both retinal and optic nerve disease. Anterior segment documentation with various imaging modalities, such as conventional ultrasound, has long been used in optometric practice. Now, with the introduction of time domain anterior segment OCT (AS-OCT) technology, including Visante OCT (Carl Zeiss Meditec) and SL-OCT (Heidelberg Engineering), you can generate in vivo, cross-sectional tissue imaging to provide diagnostic and management capabilities unlike ever before. Prior to the introduction of this technology in 2006, the only way to obtain cross-sectional images of the anterior segment was with histological sectioning.
Since its introduction more than 20 years ago, posterior segment imaging with optical coherence tomography has become the standard of care for diagnosing and managing both retinal and optic nerve disease. Anterior segment documentation with various imaging modalities, such as conventional ultrasound, has long been used in optometric practice. Now, with the introduction of time domain anterior segment OCT (AS-OCT) technology, including Visante OCT (Carl Zeiss Meditec) and SL-OCT (Heidelberg Engineering), you can generate in vivo, cross-sectional tissue imaging to provide diagnostic and management capabilities unlike ever before. Prior to the introduction of this technology in 2006, the only way to obtain cross-sectional images of the anterior segment was with histological sectioning.
This article provides an introduction to AS-OCT and explores several of its clinical applications, including glaucoma, iris and corneal examination, as well as both pre- and postoperative refractive evaluation.
An Introduction to AS-OCT
In 1994, Joseph Izatt, Ph.D., and associates first performed an anterior segment evaluation with OCT using a 1310nm wavelength.1,2 (By comparison, posterior segment OCT uses an 820nm wavelength to produce cross-sectional images of the retina and optic nerve.) The 1310nm wavelength of AS-OCT has several advantages over an 820nm wavelength. First, it permits decreased retinal exposure because water in the ocular tissue absorbs the light energy.3 Secondly, it gathers data at speeds 20 times faster than the 820nm wavelength at the same signal-to-noise ratio, which minimizes patient motion artifacts and improves the quality of captured images. And, finally, a 1310nm wavelength reduces the amount of signal scattering, which allows better penetration into turbid tissue, such as the sclera, iris and anterior chamber angle.4,5 In comparison to ultrasound biomicroscope (UBM), AS-OCT is more user-friendly and allows for non-contact acquisition of images with near-infrared light vs. water immersion image acquisition with sound waves. The axial resolution of the AS-OCT is 18µm, compared to 50µm for the UBM.
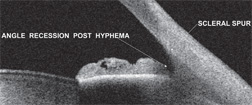 |
| 1. A patient with angle recession. Note a marked posterior displacement of the iris and an abnormally deep chamber angle. |
Specific software tools not only allow you to quantify the angle in question, but also recognize other angles that potentially are at risk. Also, you can observe and document angle progression over time as a patient’s crystalline lens thickens. Software applications include angle and iridocorneal tools. The angle tool quantifies the width of the angle in degrees. Angles less than 15° are considered “at risk” for angle closure. One study found that the sensitivity and specificity rate of anterior segment OCT angle assessment vs. traditional gonioscopy ranged from 85% to 90% respectively.7 The iridocorneal tools allow for easy calculation of measurements that were previously derived with the UBM. When the operator selects the iridocorneal tool, a template angle is produced. Then, the user positions the tool marker at the scleral spur. Next, the software automatically calculates the angle opening distance (AOD), the angle recess area (ARA), the trabecular-iris space area (TISA) and the trabecular-iris contact length (TICL). At present, these extrapolated measurements do not have much practical utility; however, in the future, clinicians might be able to use the information to screen for angle-closure glaucoma more precisely.8
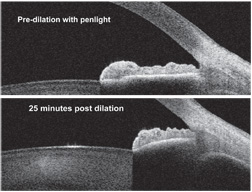 |
| 2, 3. Grade 4+ open chamber angle before dialation. Note that the
scleral spur and the trabecular meshwork are fully exposed (top).
Twenty-five minutes after dilation, the scleral spur is occluded, but
the trabecular meshwork is still exposed (bottom).
|
• Iris evaluation. AS-OCT allows for observation of the anatomical relationship of the iris, its root, and the angle anatomy at the corneoscleral junction. By isolating the scleral spur and Schwalbe’s line, the anatomical relationship between the trabecular meshwork and the iris can be visualized. Without indentation from the gonio lens, there are no compression artifacts or false impressions of either angle width or depth, nor are there any anatomical variations of the iris. Evaluation of these anatomical relationships, with or without a light source, allows you to assess various iris and anterior chamber morphologies during physiologic changes, which is not possible on gonioscopy. Specifically, you can obtain virtual biopsies of plateau iris syndrome, reverse pupillary block in pigmentary dispersion syndrome and angle recession.
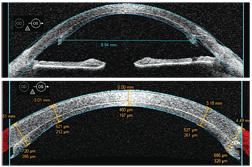
• Refractive surgery. AS-OCT has dual applications in refractive surgery. Visante OCT, for example, provides global corneal thickness measurements for preoperative planning and offers a difference map feature that illustrates changes in postoperative corneal thickness. Secondly, AS-OCT indicates flap architecture and thickness as well as residual bed thickness, which is particularly helpful in considering re-treatments for patients who were clinically assessed as borderline for LASIK enhancement preoperatively and in diagnosing unexpected postoperative cases of ectasia secondary to thicker-than-expected flaps. Both Visante OCT and SL-OCT include a corneal flap tool that documents flap thickness and residual bed thickness along the entire ablation zone. The numbers above the cornea have a “+” for values positioned to the right of center or a “-” for values left of center. The number pairs below the cornea represent the thickness of the flap (top number) and the residual bed thickness (bottom number). (See “Corneal Flap Thickness Determined With AS-OCT.”)
| Corneal Flap Thickness Determined With AS-OCT | ||
| •
Patient history. A 48-year-old white female presented for a routine
examination with a chief complaint of decreased vision in her right
eye. Her ocular history was significant for LASIK five years earlier.
• Diagnostic data. Our patient’s entering central corneal thickness measured 540µm O.D. Manifest refraction was -6.00D. Topography was unremarkable, with K values of 43.75 x 44.12. • Discussion. We created a 180µm flap with a microkeratome and performed a traditional ablation procedure with the VISX Star S4 excimer laser. The procedure was performed without incident, and the laser printout showed that 86µm of tissue was ablated. The patient did well postoperatively, with an uncorrected visual acuity of 20/20 and a refraction of -0.25D sphere. But, during the next five years, we began to see a steepening of the cornea inferiorly with irregular astigmatism. The AS-OCT image reveals a central corneal flap thickness of 219µm and a residual bed thickness of 215µm. This clearly breaches the standard of care for residual bed thickness of 250µm.
|
The pachymetry maps provide global thickness measurements from the central cornea out to 10mm. Minimum, average and maximum thickness values are provided for each area. This application also documents global changes in corneal thickness, which can be used to confirm or deny expected laser ablation results as well as diagnose post-LASIK edema. One study evaluated repeatability and reproducibility of central corneal thickness (CCT) measurements obtained by both the Visante OCT and SL-OCT.9 When compared to ultrasound pachymetry readings, both Visante-OCT and SL-OCT demonstrated reliable, reproducible CCT measurements.
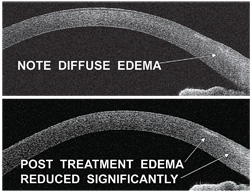 |
| 5, 6. This patient, who overwore his
contact lenses, demonstrates limbal congestion and edema as well as
multiple peripheral subepithelial infiltrates (SEIs) (top). But,
congestion and limbal edema resolved following treatment of contact
lens overwear. Note the residual scarring from SEIs (bottom).
|
Then, in 2007, Joshua C. Teichman, M.D., and associates considered a modification of the CLR measurement with respect to narrowing of the anterior chamber angles. Often, patients with large cataracts can suffer some degree of elevated intraocular pressure from narrowing of the angle.8 By assessing the amount of lens rise relative to anterior chamber depth, you can determine the probability of opening the angle by removing the crystalline lens vs. laser peripheral iriditomy (LPI). Parameters that indicate lensectomy over LPI include a CLR of 0.8mm, a chamber depth less than 2mm, and angles less than 15°.
• Corneal pathologies. AS-OCT is very useful for assessing corneal pathologies. It can be used to determine the exact depth of foreign body penetration or the existence of a residual defect following removal. Additionally, AS-OCT can be used to diagnose and manage corneal infiltrate, ulcer or dellen healing, as well as to document the extent of Fuchs’ dystrophy. Demonstrating an in vivo, cross-sectional illustration of the patient’s eye enhances his or her comprehension of the disease process, which ultimately can improve compliance.
 |
| 7, 8. A donor graft
on a postoperative DSAEK patient (top). AS-OCT differentiates the
thickness of the patient’s corneal tissue and the graft at various
areas (bottom).
|
• Corneal dystrophies. Corneal dystrophies encompass a broad range of disorders that affect one or more of the corneal layers. AS-OCT provides exceptional imagery of the cornea, revealing a clear differentiation of the epithelium, stroma and endothelium. AS-OCT can help diagnose keratoconus with pachymetry mapping as well as demonstrate corneal ectasia through cross-sectional imaging in moderate and advanced cases. In advanced cases, Fuchs’ endothelial dystrophy can lead to bullous keratopathy. Corneal guttata associated with Fuchs’ dystrophy, as well as findings consistent with corneal edema and bullous keratopathy, can be imaged on AS-OCT. Also, AS-OCT can document corneal topography both before and after a patient undergoes Descemet’s stripping automated endothelial keratoplasty (DSAEK) for Fuchs’ dystrophy or pseudophakic bullous keratopathy (figures 7 and 8).12
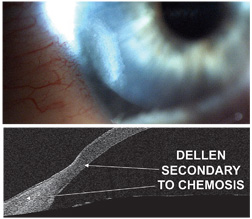 |
| 9, 10. A digital image (top) and an AS-OCT image (bottom) of a corneal dellen secondary to conjunctival chemosis.
|
We have just begun to realize and appreciate the many applications of AS-OCT. Like posterior segment OCT, clinicians are now recognizing the significance of AS-OCT for diagnosing, monitoring progression and clinical decision-making. Only when eye care clinicians become familiar with AS-OCT will the true potential of this technology be fully realized.
Dr. Miller is in private practice in Stillwater, Okla.. He is a member of the American Optometric Association and the Oklahoma Association of Optometric Physicians. He lectures on behalf of Carl Zeiss Meditec.
1. Izatt JA, Hee MR, Swanson EA, et al. Micrometer scale resolution imaging of the anterior eye in vivo with optical coherence tomography. Arch Ophthalmol. 1994 Dec;112(12):1584-9.
2. Radhakrishnan S, Rollins AM, Roth JE, et al. Real-time optical coherence tomography for the anterior segment at 1310nm. Arch Ophthalmol. 2001 Aug;119(8):1179-85.
3. Van den Berg TJ, Spekreijse H. Near infrared light absorption in the human eye media. Vision Res. 1997 Jan;37(2):249-53.
4. Huang D, Li Y, Radhakrishnan S. Optical coherence tomography of the anterior segment of the eye. Ophthalmol Clin North Am. 2004 Mar;17(1):1-6.
5. Radhakrishnan S, Huang D, Smith SD. Optical coherence tomography imaging of the anterior chamber angle. Ophthalmol Clin North Am. 2005 Sep;18(3):375-81, vi.
6. Lee R, Ahmed IK. Anterior Segment Optical Coherence Tomography: Noncontact High Resolution Imaging of the Anterior Chamber. In: Techniques in Ophthalmology. Philadelphia: Lippincott, Williams & Wilkins, 2006:120-7.
7. Wirbelauer C, Karandish A, Haberle H, Pham DT. Noncontact goniometry with optical coherence tomography. Arch Ophthalmol. 2005 Feb;123(2):179-85.
8. Teichman JC, Ahmed IIK. AS-OCT upgrades advance glaucoma care. Rev Ophthalmol E-News. 2007 Nov;14(11): 1-4.
9. Li H, Leung CK, Wong L, et al. Comparative study of central corneal thickness measurement with slit-lamp optical coherence tomography and Visante optical coherence tomography. Ophthalmology. 2008 May;115(5):796-801.e2.
10. Baikoff G, Bourgeon G, Jodai HJ, et al. Pigment dispersion and Artisan phakic intraocular lenses: crystalline lens rise a safety criterion. J Cataract Refract Surg. 2005 Apr;31(4):674-80.
11. Schafer J. Contact lens-related microbial keratitis: A look at MK causes, signs and symptoms, management strategies, differential diagnoses and patient education. Cont Lens Spect. 2007 Aug;22(8):41-8.
12. Terry M, Chen E, Shamie N, et al. Endothelial cell loss after Descemet’s stripping endothelial keratoplasty in a large prospective series. Ophthalmology. 2008 Mar;115(3):488-496.e3.
13. Casser L, Lingel NJ. Diseases of the cornea. In: Bartlett JD, Jaanus SD (eds). Clinical Ocular Pharmacology, 3rd ed. Boston: Butterworth-Heinemann, 1995:679-745.
14. Kabat AG, Sowka JW. Corneal Atlas: Part IV. How to detect and deal with dystrophies and degenerations. Rev Optom. 1999 Nov;136(11):60-71.


