 |
A 62-year-old Black male presented to the emergency department with complaints of redness and soreness of the left eye for one week with associated subjective ipsilateral vision loss. The patient’s entering visual acuity was 20/25 with pinhole in both eyes. His intraocular pressures (IOPs) were 17mm Hg OD and 16mm Hg OS. The right pupil was normal, but the left was slightly dilated, sluggish and minimally reactive to light. There was no afferent pupillary defect appreciable by reverse.
Slit lamp examination of the right eye was largely unremarkable, but the conjunctiva of the left eye exhibited diffuse injection (Figure 1). The left eye did not have any neovascularization of the iris, and the globe was not tender to palpation. Both eyes had clear corneas and quiet anterior chambers. There was bilateral optic nerve cupping consistent with a known diagnosis of bilateral primary open-angle glaucoma. The right fundus had bear tracking pigmentation. The left fundus, however, was abnormal with diffuse retinal blot hemorrhages throughout the arcades and peripherally. The retinal arteries in the left eye were attenuated and the veins were engorged, with slight tortuosity (Figure 2).
The patient had a history of cataracts and glaucoma, for which he was using netarsudil/latanoprost, brimonidine/timolol and dorzolamide in both eyes with reported compliance. At his most recent exam two months prior, his IOPs were 18mm Hg OD and 33mm Hg OS on the same medications, revealing a peculiar IOP reduction in the left eye. At that same visit, the fundus exam was reportedly normal without hemorrhages. He had been referred to a glaucoma surgeon due to uncontrolled glaucoma on maximum medical therapy in the left eye. His systemic medical history was significant for medically managed diabetes, hypertension, hyperlipidemia and human immunodeficiency virus.
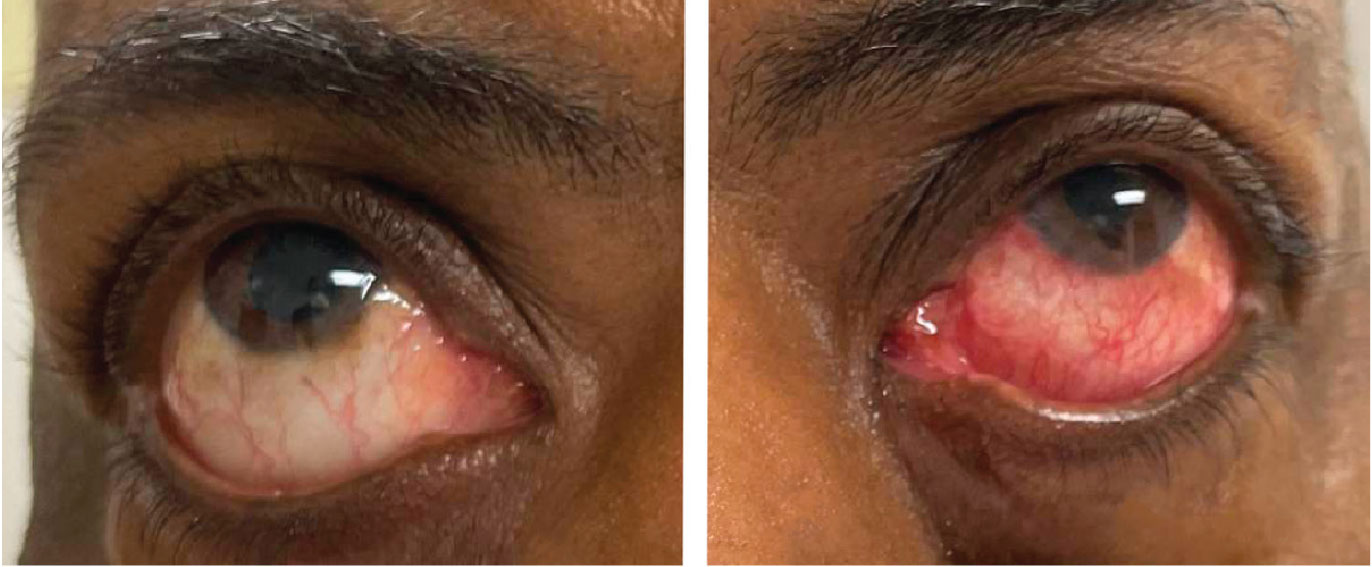 |
| Fig. 1. Note the asymmetry in conjunctival injection between the right and left eyes. Images were taken after the instillation of tropicamide and phenylephrine drops. Click image to enlarge. |
Fundus Findings
Retinal hemorrhages can present due to several conditions including, but not limited to, diabetes, hypertension, sickle cell disease and hematologic malignancy. Hemorrhaging is bilateral and relatively symmetric in these conditions. Unilateral retinal hemorrhages, however, are most commonly seen in retinal vein occlusion, which may involve the central retinal vein (CRVO) or any branch tributary.1 In the acute phase, a fundus exam typically reveals optic nerve edema, diffuse flame and blot hemorrhages in all four quadrants and dilated, tortuous veins along the affected distribution. It is common to also see concurrent cotton wool spots and cystoid macular edema (CME).
While the clinical appearance of our patient’s fundus did have some features seen in CRVO, the distribution and appearance of the retinal hemorrhages was somewhat atypical. Specifically, there were no flame hemorrhages, and the hemorrhages were mostly located outside the posterior pole. We could have considered a resolving CRVO, in which case the optic nerve edema and retinal hemorrhages could have already cleared, but the patient had seen his ophthalmologist for a dilated eye exam two months prior, and no retinal hemorrhages were noted at that time. It was therefore less likely that this was an old CRVO. Furthermore, was the conjunctival injection and ocular pain simply a red herring?
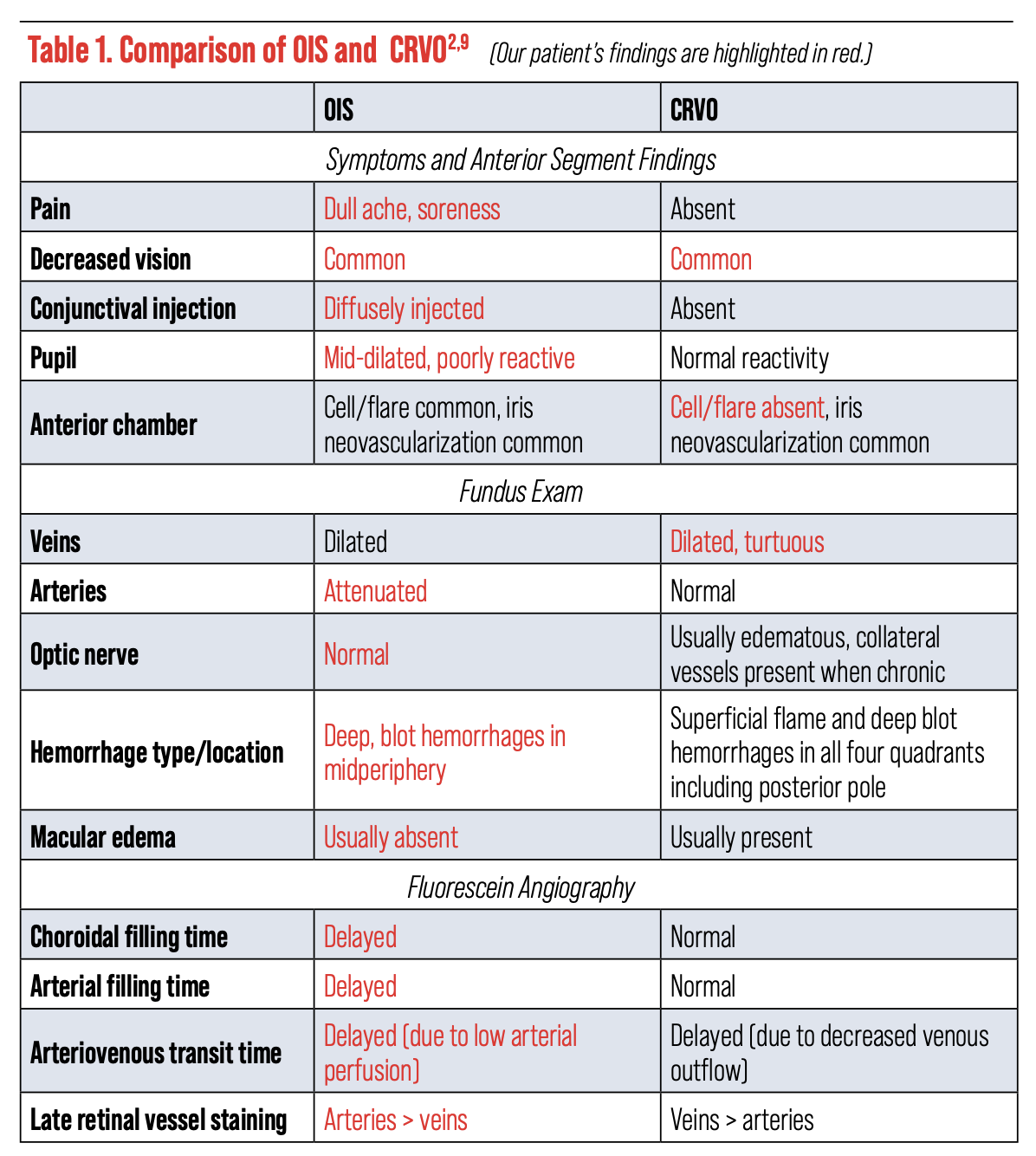
Patients can have multiple ocular conditions simultaneously, so first simplify the situation by considering a unifying condition that could produce all the ophthalmic findings in question. Ocular ischemic syndrome (OIS) is a rare condition that presents at a rate of about 7.5 cases per million individuals every year.2 It often appears as a painful red eye, minimally reactive pupil, anterior chamber inflammation and iris neovascularization with low IOP. Furthermore, the dilated fundus exam reveals midperipheral blot hemorrhages, dilated veins and attenuated arteries. Optic disc edema and macular edema are uncommon.
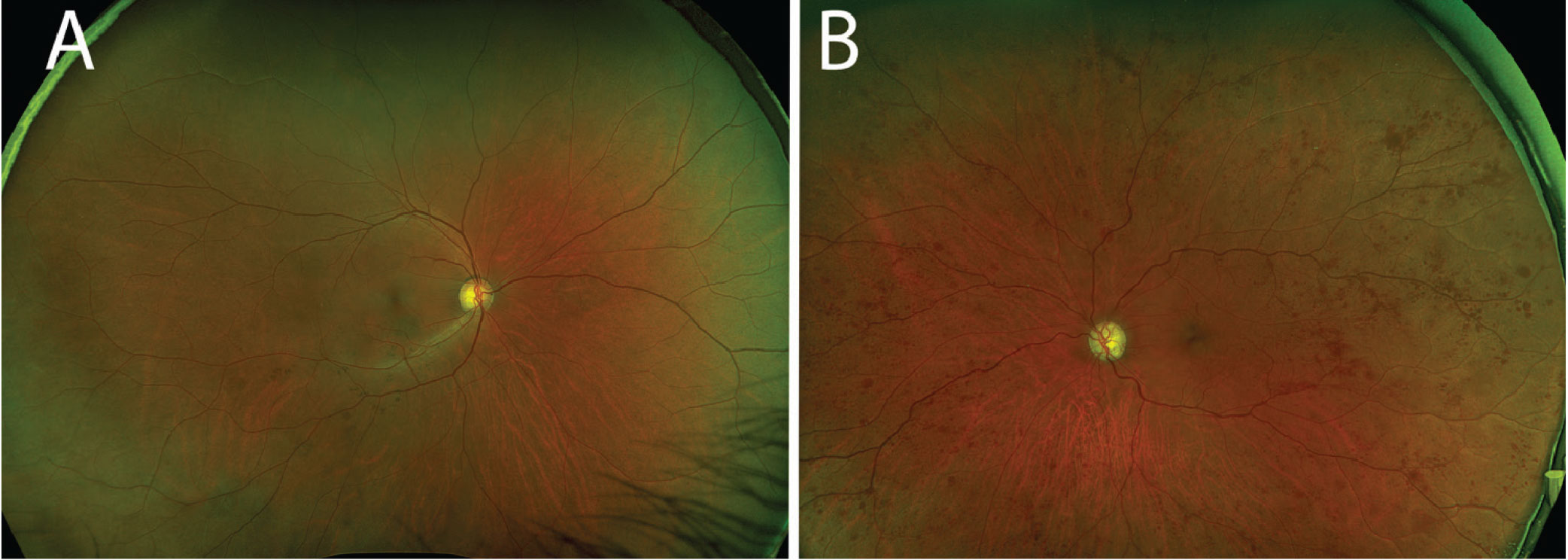 |
Fig. 2. (A) The right fundus was largely unremarkable. (B) In the left fundus, one can appreciate the extensive blot hemorrhages in the midperiphery and periphery. Note the attenuated arteries and dilated veins. Click image to enlarge. |
Can We Break the Tie?
Though our patient did not have anterior chamber inflammation or neovascularization at his initial visit, his exam was still concerning for OIS. Given the overlap in clinical presentation of CRVO and OIS, consider the imaging modalities available to distinguish between the two conditions. First, recall that CME is much more common in CRVO than OIS, presenting in the majority of CRVO and only 15% to 17% of OIS cases.2,3 OCT—helpful in evaluating the macular status—revealed an absence of CME (Figure 3).
Another tool that can aid in distinguishing these two conditions is intravenous fluorescein angiography (FA). In OIS, this method often reveals delayed choroidal flush, delayed arm-to-retina time, prolonged retinal circulation time and late staining of the arteries.2
Our patient’s FA was ordered with the left eye as “transit,” meaning that photos of the left fundus were taken as soon as fluorescein dye was pushed into the circulation through the antecubital vein. Choroidal flush is the first angiographic sign seen in FA. Normal choroidal flush occurs at about 10 to 15 seconds.4,5 Our patient’s imaging revealed a delayed choroidal filling, with choroidal flush first appearing beyond the normal threshold at 26 seconds (Figure 4a). Arterial circulation was also delayed and only first appreciable at 35 seconds, nine seconds after the choroidal flush (normal: one to two seconds after).
Later images revealed incomplete filling of the arterial branches signifying retinal arterial stasis (Figure 4b). Additionally, late-phase imaging at five minutes revealed staining of the retinal arteries without venous staining or leakage, which was felt to be more consistent with an arterial process.
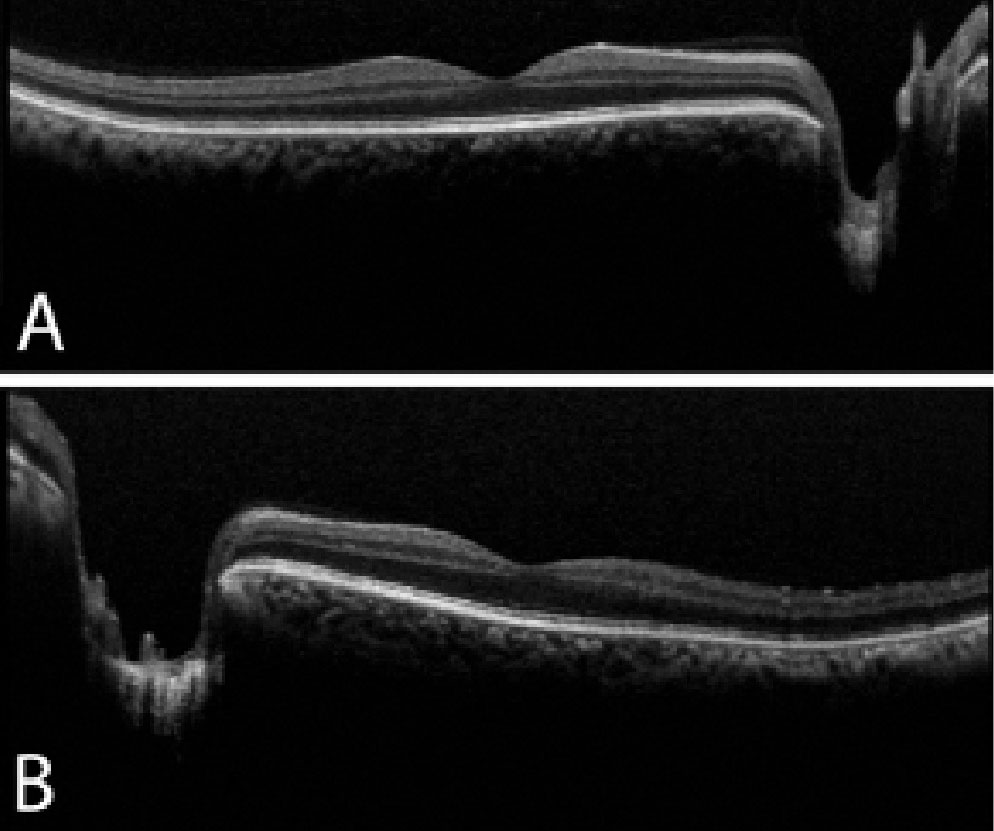 |
| Fig. 3. OCT of the right (A) and left (B) eyes reveals no macular edema or retinal thickening. There is deep cupping in the left eye greater than the right eye, which corroborates with the fundus exam. Click image to enlarge. |
Putting it All Together
Once a diagnosis of OIS is on the table, the clinician’s job is not complete. It is important to discuss the condition fully with the patient and communicate findings with the patient’s other healthcare providers. OIS is most commonly seen in cases of severe carotid artery occlusion or stenosis. It has also been attributed to vasculitis (such as giant cell arteritis and Takayasu arteritis), aortic arch syndrome, carotid artery dissection and hyperhomocysteinemia.2,6,7
 |
| Click table to enlarge. |
The recommended lab studies for an OIS workup include erythrocyte sedimentation rate, C-reactive protein and complete blood count. Imaging studies of the carotid arteries and heart—such as a carotid duplex and ultrasound, angiography of the head and neck and an echocardiogram—should also be obtained. Multispecialty management is always indicated in these cases, and patients should be promptly referred to a primary care physician, cardiologist and/or neurologist for a complete workup. They may be advised to seek treatment with a vascular surgeon or interventional radiologist in the case of significant carotid stenosis. Treatment will ultimately depend on the underlying condition and its severity as well as the patient’s comorbidities.
Patients with OIS are at risk of developing severe ocular complications such as neovascularization and neovascular glaucoma. They should be monitored regularly with gonioscopy and dilated fundus examination to evaluate for any signs of neovascular changes. If present, treatments such as panretinal photocoagulation and intravitreal anti-VEGF injection should be considered, though the overall visual prognosis remains poor in cases of OIS.8
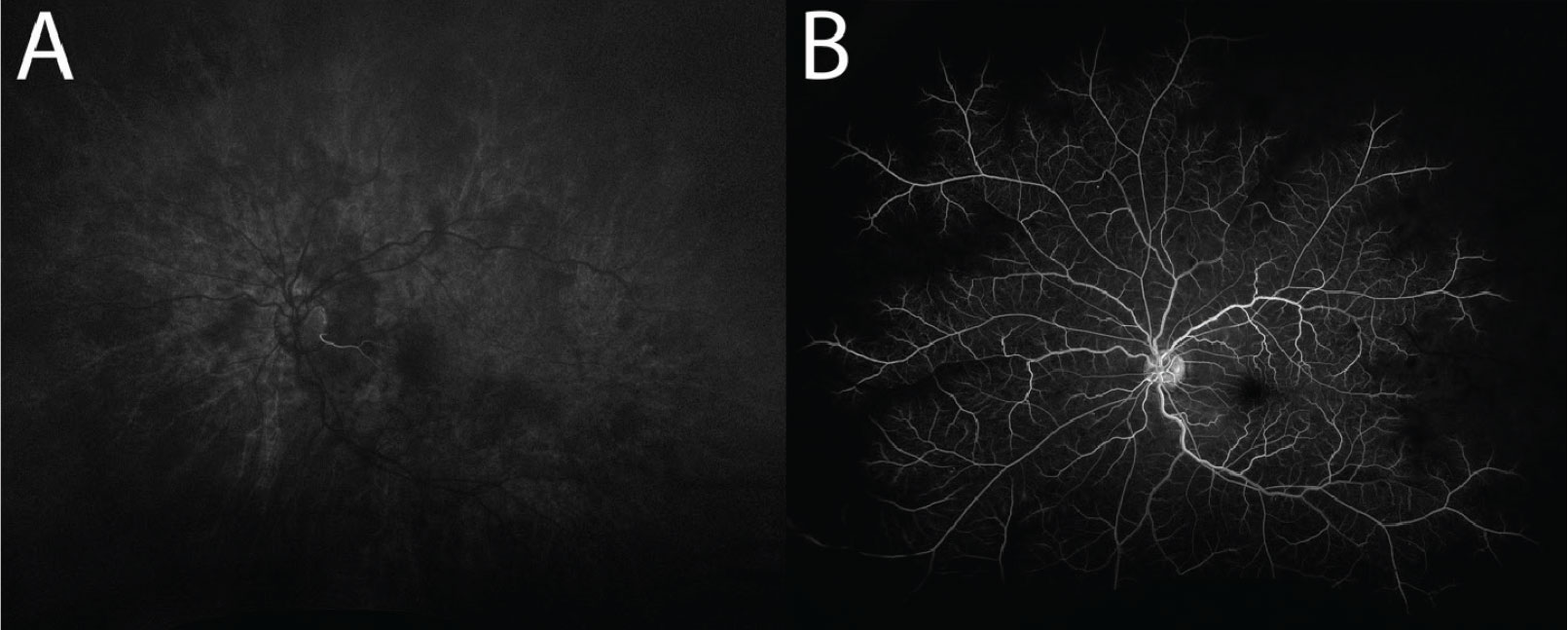 |
Fig. 4. (A) Delayed first appearance of choroidal flush, captured at 26 seconds. Note the filling of a small cilioretinal artery, which is supplied by the choroid. (B) Delayed and incomplete filling of the retinal arteries, captured at 69 seconds. There are also a few microaneurysms present. Click image to enlarge. |
Management and Status
The patient was under the care of a cardiologist and was urgently advised to seek further workup. Unfortunately, as of the most recent follow-up, he was still awaiting carotid and cardiac studies to confirm or rule out the suspected diagnosis. He continued to have persistent ocular redness and pain and developed iris neovascularization. The IOP and retinal findings remained stable. Due to the appearance of neovascular changes, he received an intravitreal anti-VEGF injection and panretinal photocoagulation in the left eye.
Dr. Bozung works in the Ophthalmic Emergency Department of the Bascom Palmer Eye Institute (BPEI) in Miami and serves as the clinical site director of the Optometric Student Externship Program as well as the associate director of the Optometric Residency Program at BPEI. She has no financial interests to disclose.
|
1. Rehak M, Wiedemann P. Retinal vein thrombosis: pathogenesis and management. J Thromb Haemost. 2010;8(9):1886-94. 2. Terelak-Borys B, Skonieczna K, Grabska-Liberek I. Ocular ischemic syndrome — a systematic review. Med Sci Monit. 2012;18(8):RA138-44. 3. Laouri M, Chen E, Looman M, Gallagher M. The burden of disease of retinal vein occlusion: review of the literature. Eye (Lond). 2011;25(8):981-8. 4. 2020-2021 Basic and Clinical Science Course, Section 12: Retina and Vitreous. American Academy of Ophthalmology, 2020:33-37. 5. Ruia S, Tripathy K. Fluorescein Angiography. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. 6. Larrazabal RB, Chiu HHC, Magbitang-Santiago AT. Takayasu arteritis presenting as bilateral ocular ischemic syndrome. Vasc Specialist Int. 2020;36(3):163-9. 7. Luo J, Yan Z, Jia Y, Luo R. Clinical analysis of 42 cases of ocular ischemic syndrome. J Ophthalmol. 2018;2018:2606147. 8. Hazin R, Daoud YJ, Khan F. Ocular ischemic syndrome: recent trends in medical management. Curr Opin Ophthalmol. 2009;20(6):430-3. 9. Mendrinos E, Machinis TG, Pournaras CJ, Ocular ischemic syndrome. Surv Ophthalmol. 2010;55(1):2-34. |

