On a fine, spring Sunday evening about 17 years ago, I was on call at our eye care group and on the phone with the daughter of an 82-year-old who had complained of eye pain early that morning. The daughter said her mother’s eye was red, vision seemed cloudy and she felt nauseous and had a headache. I remember giving her a directive: “Look at your mom’s pupils and see if they look different.” She returned and said, “Yes, the eye that’s red has a larger pupil.” Individually, each symptom could take the clinician down many paths. Taken together, an acute angle closure must be strongly considered. I told her to meet me at the office in 15 minutes.
Fortunately, with a prompt diagnosis we were able to break that patient’s angle-closure attack and get her in for a laser peripheral iridotomy (PI) quickly.
Because most optometrists will see an angle-closure attack similar to this at some point, an understanding of the current thinking in the management of narrow-angle and angle-closure glaucoma and how optometrists are poised to stabilize and manage these patients is vital.
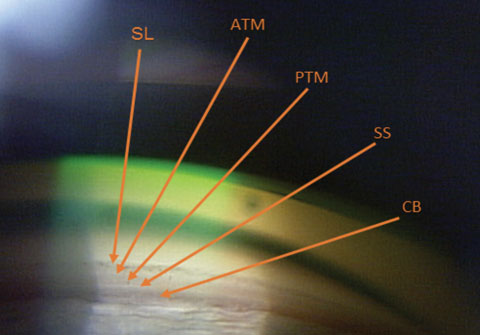 |
| Fig. 1. The ciliary body (CB) is the most posterior structure visible, followed by the scleral spur (SS), posterior trabecular meshwork (PTM), anterior trabecular meshwork (ATM) and, finally, Schwalbe’s line (SL). |
Angle-closure Up Close
Angle-closure glaucoma (ACG) affects 20 million people worldwide, and about four million are bilaterally blind.1 Reports suggest the total number of people between the ages of 40 and 80 affected by angle-closure glaucoma will increase to 23 million by 2020 and 32 million by 2040.2 ACG causes nearly half of all glaucoma blindness worldwide.3 Even though there are three times more people worldwide with primary open-angle glaucoma (POAG) than ACG, angle-closure’s increased morbidity causes blindness in about the same number.4
Patients undergoing an acute symptomatic angle-closure attack present with symptoms of ocular or periocular pain, reduced vision with halos, eye redness and nausea or vomiting. Ocular signs include elevated intraocular pressure (IOP), corneal edema, mid-dilated pupil, shallow anterior chamber and conjunctival injection with ciliary flush.3
There are three currently accepted categories for angle-closure disease: primary angle-closure suspect, primary angle-closure and angle-closure glaucoma.5-7 An ACG suspect has an angle where the trabecular meshwork cannot be seen for half or more of the angle gonioscopically, indicating at least 180 degrees of iridotrabecular contact. These patients will not have peripheral anterior synechiae, which are the result of long-term iridotrabecular contact.8 A primary angle-closure patient will have a closed angle with a rise in IOP, possibly with peripheral anterior synechiae. Patients with ACG will have a closed angle, peripheral anterior synechiae and evidence of glaucomatous damage in either the disc or field. The glaucomatous damage of the nerve in patients with ACG is similar in nature to glaucomatous damage in patients from POAG, while the field defect may be more diffuse in ACG. 9
Demographic risk factors include female gender, advanced age and Asian ancestry.10-12 Ocular risk factors include narrow angles, shallower axial and limbal anterior chamber depth, thicker lens, shorter axial length, more anteriorly positioned lens, smaller corneal diameter and hyperopic refraction.3 Population-based studies suggest a genetic component, but the exact genetic pattern remains elusive.13
Case 1A 55-year-old white female presented with intense pain in the left eye. She reported it began the previous night shortly before bed and has progressed since. She now reports the pain as 11 on a scale of one to 10. Her ocular history is significant for an optic nerve coloboma in the left eye.
Visual acuity was 20/20 OD and light perception (LP) OS because of the coloboma. She reported seasonal allergies controlled with Claritin (loratadine, Bayer). Goldmann tonometry was 20mm Hg OD and 56mm Hg OS. Biomicroscopy OS revealed corneal edema, grade 1 cells in anterior chamber, grade 1 Van Herick and a dense, grade 4 nuclear sclerotic cataract. Gonioscopy revealed a closed angle with no view of TM and no peripheral anterior synechiae. Anterior segment angle OCT confirmed angle closure (Figure 2). We diagnosed her with phacomorphic acute angle closure. We instilled one drop of Iopidine (0.5% apraclonidine hydrochloride, Alcon), followed a few minutes later by one drop of Cosopt (dorzolamide HCL/0.5% timolol maleate ophthalmic solution, Merck). This was repeated 20 minutes later. We also gave the patient 500mg acetazolamide PO. Approximately one and a half hours after diagnosis, the patient’s IOP was 47mm Hg. Lacking isosorbide, we performed compression gonioscopy, which lowered IOP to 34mm Hg. A repeat OCT angle was still quite narrow, but open (Figure 3). We scheduled the patient for immediate laser PI OS with subsequent cataract surgery a few days later. Her pressure is now stable in the 15mm Hg to 17mm Hg range OS. |
Anatomy
Although an underused procedure, gonioscopy remains the standard for viewing the angle and making the diagnosis of angle-closure. One study found that less than half of all eye care providers performed gonioscopy on their glaucoma patients.14 Understanding the anatomy is crucial to help identify variances associated with ACG (Figure 1).
In an open angle, the most posterior structure visible is the ciliary body (CB), which is found between the iris root and the scleral spur. It can vary from light gray to brown and may reduce complete visualization. The second most posterior structure, the scleral spur, is found in the posterior margin of the scleral sulcus, between the CB and the trabecular meshwork (TM). It is made up of collagen tissue, serves as the anchor for the ciliary muscle and can vary in color from white to gray. The TM is next, found between the scleral spur and Schwalbe’s line. It can be subdivided into anterior and posterior TM. It is typically light gray in younger patients and becomes more pigmented over time. The anterior third of the TM is nonfunctional, while the posterior two-thirds filters aqueous into Schlemm’s canal. Schwalbe’s line is the most anterior angle structure and represents the end of a clear cornea.
Table 1. Scheie Classification System15 | |
There are three main classification systems—Scheie, Shaffer and Spaeth (Tables 1-3) for ACG—each with its own strengths and weaknesses.15-17 In general, using these grading systems may complicate comanagement between clinicians, as a grade 1 can mean two vastly different angle configurations. A good rule is always to describe the last structure seen.
Mechanisms
Angle-closure refers to the appositional closure of the anterior chamber angle, resulting in aqueous obstruction. The most common underlying mechanism of primary angle-closure is pupillary block, in which the aqueous forces the pupil forward.18 The term primary means there is no detectable cause. Ninety percent of all US patients presenting with angle-closure have pupillary block.6 Pupillary block occurs when the pressure of the posterior chamber exceeds the pressure of the anterior chamber, pushing the peripheral and midperipheral iris forward and blocking the TM. The second mechanism of primary angle-closure is plateau iris, which occurs when the CB is anterior or rotated forward, displacing the peripheral iris into the TM.6
Secondary angle-closure occurs by a known pathology. An example of a secondary angle-closure is phacomorphic glaucoma, which occurs when the lens pushes the iris forward and closes the angle.19 This may also occur in subluxation. Uveitis may cause a secondary pupil block, which is characterized by iris bombe and posterior synechiae. Other secondary causes include neovascularization, malignant glaucoma, retinopathy of prematurity, posterior scleritis, acquired immunodeficiency syndrome, Vogt-Koyanagi-Harada syndrome, leukemia, orbital or carotid cavernous fistula and neuropathia epidemica.20
Clinicians should also be aware of masqueraders such as: glaucomatocyclitic crisis, steroid-induced glaucoma, phacolytic glaucoma, ghost cell glaucoma, hemolytic glaucoma, hemorrhagic glaucoma and exfoliation glaucoma.20
Table 2. Shaffer Classification System16 | |||
| ≥10 | |||
Pharmacologic Causes
Numerous prescription and OTC medications may induce angle narrowing or angle closure. Such medications may cause up to 33% of all angle-closure attacks.21 Some of these drugs, including cholinergics such as Salagen (pilocarpine HCl, Pfizer) and Evoxac (cevimeline hydrochloride, Daiichi Sankyo), move the lens-iris diaphragm forward. Iris dilation may occur from antidepressants such as Paxil (paroxetine, GlaxoSmithKline) and Prozac (fluoxetine, Eli Lilly), incontinence medications such as Ditropan (oxybutynin, Janssen), Detrol (tolterodine tartrate, Pfizer) and Sanctura (trospium chloride, Allergan), and antihistamines such as Tagamet (cimetidine, Prestige), Zantac (ranitidine HCl, Boehringer Ingelheim) and Benadryl (diphenhydramine, Johnson & Johnson). There are safer nonsedating H1 blocking antihistamines such as loratadine, cetirizine and fexofenadine that are less likely to cause ACG.22
Another potential inducer of secondary angle-closure is Topamax (topiramate, Janssen). Topamax is indicated for the treatment of epilepsy and migraines; it is also used off-label to treat post-traumatic stress disorder and alcohol addiction. It can cause swelling of the ciliary body and lens, anterior rotation of the lens-iris diaphragm and bilateral angle closure, as well as uveitis and sudden myopic shift.23 Up to 89% of Topamax-induced angle closures occur in women.24 Management of a Topamax angle-closure consists of immediately discontinuing the medication, as well as initiating medical IOP reduction, cycloplegics, topical and intravenous steroids and intravenous Osmitrol (mannitol, Baxter Healthcare Corporation). Usually it resolves in one week with this treatment.25 Unlike in primary angle-closure, laser PI is ineffective, as Topamax angle-closure does not involve pupillary block.
When an astute primary care provider calls to discuss putting a mutual patient with glaucoma on one of these medications, bring the patient in for gonioscopy and OCT angle imaging prior to the initiation of the new medication. Educate the patient on the signs and symptoms of angle-closure glaucoma and repeat testing three to four weeks after initiating the medication to ensure it has not induced angle-closure glaucoma.
Table 3. Spaeth Classification System17 | |
Level of iris insertion | A: Anterior: iris inserts anterior to SL B: Behind Schwalbe’s line: anterior to posterior limit of the TM, or between SL and SS C: Sclera: posterior to SS. SS is visible D: Deep: deep into the CB E: Extremely deep: very deep into the CB |
| Angular width | Estimated angle in degrees |
| Iris configuration | B: (steep) bowing anteriorly, graded on a 1-4+ scale P: plateau configuration F: flat configuration C: concave, posterior bowing |
| Pigment grading | Pigment in PTM at 12 o’clock position graded on a 0-4+ scale |
Diagnostic Tools
While gonioscopy remains the standard for diagnosing angle-closure, angle OCT and ultrasound biomicroscopy (UBM) are playing an increasingly important role. Both of those technologies can give an objective assessment of the angle width.22 Angle OCT is non-contact, is more tolerable to the patient and provides better resolution. UBM can image the CB more clearly because of deeper sound wave penetration. In the same way that posterior segment OCT imaging may not be optimal for visualizing characteristics such as small drance-type optic nerve hemorrhages, angle OCT and UBM may not be adequate for distinguishing between peripheral anterior synechiae and iridotrabecular contact. While these imaging technologies may eventually become a replacement for gonioscopy, currently they are more of an adjunct.
Treatment
The first goal in the management of ACG is IOP reduction. The second goal is reversing the mechanism of angle closure.
Topical
Often used as initial treatment, eye drops that can quickly reduce IOP include beta blockers, alpha agonists, carbonic anhydrase inhibitors and pilocarpine.26 Beta blockers, alpha agonists and carbonic anhydrase inhibitors all quickly reduce aqueous production, making them ideal to use when rapid IOP reduction is desired. Pilocarpine constricts the pupil, which is helpful for subsequent laser PI. Even though pilocarpine increases the angle width in patients with narrow angles, it may actually narrow the angle in eyes with phacomorphic glaucoma, pseudoexfoliation and vitreous block glaucoma.27-29 Prostaglandins may not be as effective because of delayed onset and may increase anterior chamber inflammation.
Oral or intravenous acetazolamide or hyperosmotics can also help relieve elevated IOP. Because quick reduction is warranted, acetazolamide sequels are less effective, as they reduce pressure slowly.
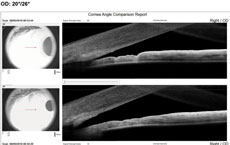
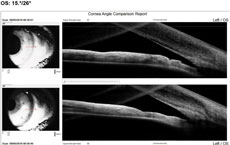
Case 2We examined a 47-year-old female with presbyopia who wears contact lenses on occasion. She reported that her mother is being followed for narrow angles. During the course of our evaluation, we graded her angle via Van Herick as grade 2. Her intraocular pressures were 21mm Hg OD and 22mm Hg OS. Her post-dilation IOPs were 22mm Hg and 23mm Hg. Her visual fields were unremarkable, and her optic nerves had a c/d ratio of 0.2/0.2 OD and OS. Her neuroretinal rims were pink and well-perfused. The angle OCT taken under scotopic conditions shows an angle graded at 20 degrees OD and 15 degrees OS (Figures 4 and 5). We performed gonioscopy and were able to see anterior ciliary body OU (Figure 6).
| ||||||
Topical steroids are helpful to relieve inflammation, and topical osmotic agents such as glycerin can reduce corneal edema and clear the cornea quickly if corneal edema is present and the anterior chamber and iris structures are difficult to clinically visualize.
Optometrists should be aware of medication contraindications, including: asthma and COPD for beta blockers; severe cardiac and cerebrovascular disease for alpha agonists; and kidney disease or sulfa allergies for carbonic anhydrase inhibitors. Clinicians must always weigh the treatment risks with the risks of nontreatment with conditions such as ACG that can rapidly cause irreversible blindness.
If medical management is unsuccessful in returning IOP to a safe level, clinicians should consider indentation or compression gonioscopy.30 When performing indentation gonioscopy, use a small footprint gonio lens and apply a significant amount of pressure. The force transferred to the angle may move the peripheral iris away from the TM, suddenly reducing IOP. This will also help determine the extent of peripheral anterior synechiae. Angles with higher amounts of peripheral anterior synechiae are more likely to fail IOP reduction attempts with medical treatment and laser PI because of the iris mechanically adhering and blocking the trabecular meshwork.31 Paracentesis may help to quickly reduce IOP and pain, but clinicians must use caution, as the anterior chamber will be shallow. Paracentesis is effective in primary angle-closure but may not be as successful in secondary angle-closure.32
Surgical
Laser PI is the mainstay of angle-closure treatment. Creating an alternate outflow pathway allows the aqueous to bypass the pupil, thus eliminating the pressure differential between the anterior and posterior chambers. The iris will then return from a convex configuration in the midperipheral and peripheral area to neutral, thus opening the angle.33 While laser PI is often successful at reducing IOP and successfully treating angle-closure glaucoma, subsequent treatment with eye drops, surgery or both is often necessary.34 Clinicians should also perform a laser PI on the fellow eye, as roughly half will have an angle-closure event within five years if left untreated.35,36 The patient should be evaluated at least one day, one week and one month after a PI procedure for angle-closure.
Many clinicians recommend cataract surgery within a few weeks of a patient successfully treated for an acute angle-closure for two reasons. First, cataract surgery opens the angle more than a laser PI.37 Second, the patient typically requires fewer pressure-lowering medications after cataract surgery.38 In fact, several studies indicate clinicians should be recommending cataract surgery in lieu of laser peripheral iridotomy.39-41 Cataract surgery may be helpful at each and every stage of angle-closure treatment. Though controversial, some are advocating refractive lens exchange in angle-closure patients with clear lenses.42 The EAGLE study (Effectiveness in Angle-closure Glaucoma of Lens Extraction) may soon give additional insight to this alternate treatment option.43
If patients are unresponsive to medical therapy or laser PI, or if the cornea prevents adequate visualization for a laser iridotomy, some clinicians recommend iridoplasty—a procedure that uses a laser to contract the peripheral iris stroma away from the angle.44,45 While there may be a role for iridoplasty in the treatment of phacomorphic and plateau iris, its overall place in the treatment of angle-closure is currently in question.7,46-48
Borderline Cases
The challenge is deciding whether or not to recommend laser PI for all patients with narrow angles. Clinicians may be tempted to do so, although management of these patients is not clear cut, as fewer than one in 20 gonioscopically narrow eyes will develop angle-closure.7 Additionally, laser PI may hasten the development of cataracts.49
We don’t we have better guidelines on when to prophylactically treat because we still have very little insight on who will and who won’t have an angle-closure attack. Unfortunately, clinicians must rely on gonioscopy—which is an imperfect test because of subjectivity—and inconsistent results with different testing variables such as illumination. Additionally, we still don’t completely understand all the variables that lead to angle-closure, and the variables we do understand have little predictive value. One variable that may hold promise in helping to better understand the mechanism of angle-closure lies in the fact that the iris squeezes aqueous from its stroma when the pupil dilates.50 The iris that holds more water upon dilation may be at a higher risk for an angle-closure attack.51 This may eventually prove to be an important measurement in clinical practice.
Although angle-closure glaucoma can be challenging, optometrists are in an optimal position to manage these patients. Timely diagnosis using gonioscopy is critical, as is IOP control. The optometrist must then either perform the laser PI (in states that permit such treatment) or promptly refer the patient for laser PI or cataract surgery.
Dr. Cymbor is a partner with Nittany Eye Associates in State College, PA, and is a member of the Optometric Glaucoma Society. He is a speaker for Optovue. Dr. Cymbor would like to thank Isaac Lindermuth, fourth-year Salus student, for compiling tables 1-3 and taking figures 1, 4, 5 and 6.
|
1. Tham YC, Li X, Wong TY, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081-90. 2. Quigley H, Broman A. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262-7. 3. Weinreb RN, Friedman DS, eds. Angle Closure and Angle Closure Glaucoma - Consensus Series Book 3. Amsterdam: Kugler Publications; 2006:1-61. 4. Quigley HA. Angle-Closure glaucoma: concepts and epidemiology. Glaucoma Today. July/August 2009:34-6. 5. Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002;86(2):238-42. 6. Ritch R, Lowe RF, Reyes A. Angle-closure glaucoma: therapeutic overview. The Glaucomas. 1996;2:1521-31. 7. Quigley HA. Understanding the Problem of Angle-Closure Glaucoma. Glaucoma Today. March/April 2015;30-1. 8. Mizoguchi T, Ozaki M, Wakiyama H, et al. Peripheral iris thickness and association with iridotrabecular contact after laser peripheral iridotomy in patients with primary angle-closure and primary angle-closure glaucoma. Clinical Ophthalmology. 2014;8:517. 9. Boland MV, Zhang L, Broman AT, et al. Comparison of optic nerve head topography and visual field in eyes with open-angle and angle-closure glaucoma. Ophthalmology. 2008 Feb 29;115(2):239-45. 10. Yamamoto T, Iwase A, Araie M, et al. The Tajimi Study report 2: prevalence of primary angle closure and secondary glaucoma in a Japanese population. Ophthalmology. 2005;112(10):1661-9. 11. Bonomi L, Marchini G, Marrafa M, et al. Epidemiology of angle-closure glaucoma. Prevalence, clinical types, and association with peripheral anterior chamber depth in the Egna-Neumarkt Glaucoma Study. Ophthalmology. 2000;107(5):998-1003. 12. Dandona L, Dandona R, Mandal P, et al. Angle-closure glaucoma in an urban population in southern India. The Andhra Pradesh Eye Disease Study. Ophthalmology. 2000(9);107:1710-6. 13. Khor CC, Do T, Jia H, et al. Genome-wide association study identifies five new susceptibility loci for primary angle closure glaucoma. Nature Genetics. 2016;48(5):556-62. 14. Fremont AM, Lee PP, Mangione CM, et al. Patterns of care for open-angle glaucoma in managed care. Arch Ophthalmol. 2003;121(6):777-83. 15. Scheie HG. Width and pigmentation of the angle of the anterior chamber: a system of grading by gonioscopy. AMA Archives of Ophthalmology. 1957;58(4):510-2. 16. Becker B, Shaffer RN. Diagnosis and Therapy of the Glaucomas. St. Louis: CV Mosby; 1965:42-53. 17. Spaeth GL. The normal development of the human anterior chamber angle: a new system of descriptive grading. Transactions of the Ophthalmological Societies of the United Kingdom. 1970 Dec;91:709-39. 18. Kumar G, Ichhpujani P, Bhartiya S, et al. The lens and angle closure. J Curr Glaucoma Practice. 2010;4(1):13-20. 19. Hung T, Chou LH. Provocation and mechanism of angle-closure glaucoma after iridectomy. Arch Ophthalmol. 1979;97(10):1862-4. 20.Tello C, Rothman R, Ishikawa H, Ritch R. Differential diagnosis of the angle-closure glaucomas. Ophthalmology Clinics. 2000;13(3):443-53. 21. Lachkar Y, Bouassida W. Drug-induced acute angle closure glaucoma. Curr Opin Ophthalmol. 2007;18(2):129-33. 22. Than T, Hardie, E. Meds that don’t mix with glaucoma patients. Rev Optom. 2002;139(10). 23. Acharya N, Nithyanandam S, Kamat S. Topiramate-associated bilateral anterior uveitis and angle closure glaucoma. Indian J Ophthalmol. 2010;58(6):557-9. 24. Thambi L, Kapcala LP, Chambers W, et al. Topiramate-associated secondary angle-closure glaucoma: a case series. Arch Ophthalmol. 2002;120:1108. 25. Brandão MN, Fernandes IC, Barradas FF, et al. Acute myopia and angle closure glaucoma associated with topiramate in a young patient. Arq Bras Oftalmol. 2009;72(1):103-5. 26. Sakai H, Shinjyo S, Nakamura Y, et al. Comparison of latanoprost monotherapy and combined therapy of 0.5% timolol and 1% dorzolamide in chronic primary angle-closure glaucoma (CACG) in Japanese patients. Journal of Ocular Pharmacology & Therapeutics. 2005;21(6):483-9. 27. Kobayashi H, Kobayashi K, Kiryu J, Kondo T. Pilocarpine induces an increase in the anterior chamber angular width in eyes with narrow angles. British journal of ophthalmology. 1999;83(5):553-8. 28. Hung L, Yang C, Chen M. Effect of pilocarpine on anterior chamber angles. Journal of Ocular Pharmacology and Therapeutics. 1995;11(3):221-6. 29. Quigley HA. Angle-closure glaucoma—simpler answers to complex mechanisms: LXVI Edward Jackson Memorial Lecture. Am J Ophthalmol. 2009;148(5):657-69. 30. Forbes M. Indentation gonioscopy and efficacy of iridectomy in angle-closure glaucoma. Trans Am Ophthalmol Soc. 1974;72:488-515. 31. Salmon JF. Long-term intraocular pressure control after Nd-YAG laser iridotomy in chronic angle-closure glaucoma. Journal of Glaucoma. 1993;2(4):291-6. 32. Cioboata M, Anghelie A, Chiotan C, et al. Benefits of anterior chamber paracentesis in the management of glaucomatous emergencies. Journal of Medicine and Life. 2014;7(2):5. 33. Jin JC, Anderson DR. The effect of iridotomy on iris contour. Am J Ophthalmol. 1990;110(3):260-3. 34. Rosman M, Aung T, Ang LP, et al. Chronic angle-closure with glaucomatous damage: long-term clinical course in a North American population and comparison with an Asian population. Ophthalmology. 2002 Dec;109(12):2227-31. 35. Snow JT. Value of prophylactic iridectomy on the second eye in angle-closure glaucoma. Trans Ophthalmol Soc UK. 1997;97:189-91. 36. Henrietta H, Chew P, Sng C, et al. A comparison of two approaches to managing acute primary angle closure in Asian eyes. Clin Ophthalmol. 2013;7(7):1205-10. 37. Hayashi K, Hayashi H, Nakao F, et al. Changes in anterior chamber angle width and depth after intraocular lens implantation in eyes with glaucoma. Ophthalmology. 2000;107(4):698-703. 38. Hata H, Yamane S, Hata S, et al. Preliminary outcomes of primary phacoemulsification plus intraocular lens implantation for primary angle-closure glaucoma. Journal of Medical Investigation. 2008;55(3,4):287-91. 39. Lam DS, Leung DY, Tham CC, et al. Randomized trial of early phacoemulsification versus peripheral iridotomy to prevent intraocular pressure rise after acute primary angle closure. Ophthalmology. 2008;115(7):1134-40. 40. Tham CC, Kwong YY, Leung DY, et al. Phacoemulsification versus combined phacotrabeculectomy in medically uncontrolled chronic angle closure glaucoma with cataracts. Ophthalmology. 2009;116(4):725-31. 41. Zhuo YH, Wang M, Li Y, et al. Phacoemulsification treatment of subjects with acute primary angle closure and chronic primary angle-closure glaucoma. Journal of Glaucoma. 2009;18(9):646-51. 42. Brown RH, Zhong L, Lynch MG. Clear lens extraction as treatment for uncontrolled primary angle-closure glaucoma. J Cataract Refract Surg. 2014;40(5):840-841. 43. Azuara-Blanco A, Burr JM, Cochran C, et al. The effectiveness of early lens extraction with intraocular lens implantation for the treatment of primary angle-closure glaucoma (EAGLE): study protocol for a randomized controlled trial. Trials. 2011;12(1):1. 44. Ritch R, Clement CY, Lam DSC, et al. Surgical techniques. Argon laser peripheral iridoplasty (ALPI): an update. Surv Opthalmol. 2007;52(3):279-88. 45. Agarwal HC, Kumar R, Kalra VK, et al. Argon laser iridoplasty: a primary mode of therapy in primary angle closure glaucoma. Indian J Ophthalmol. 1991;39(3):87. 46. Tham CC, Lai JS, Poon AS, et al. Immediate argon laser peripheral iridoplasty (ALPI) as initial treatment for acute phacomorphic angle-closure (phacomorphic glaucoma) before cataract extraction: a preliminary study. Eye. 2005;19(7):778-83. 47. Ritch R, Tham CC, Lam DS. Long-term success of argon laser peripheral iridoplasty in the management of plateau iris syndrome. Ophthalmology. 2004;111(1):104-8. 48. Friedman D, Lin S, Leibman D. Managing narrow angles and glaucoma. Rev Ophthalmol. 2013;20(6):23-32. 49. Lim LS, Husain R, Gazzard et al. Cataract progression after prophylactic laser peripheral iridotomy: potential implications for the prevention of glaucoma blindness. Ophthalmology. 2005;112(8):1355-9. 50. Quigley HA, Friedman DS, Congdon NG. Possible mechanisms of primary angle-closure and malignant glaucoma. J Glaucoma. 2003;12(2):167-80. 51. Barkana B, Tham CC, Dorairaj SK, et al. Angle-closure glaucoma: posterior (pushing) mechanisms without pupillary block. In: Tombran-Tink J, Barnstable CJ, Shields MB, eds. Ophthalmology Research: Mechanisms of the Glaucomas. Totowa, NJ: Humana Press/Springer; 2008:173-87. |