 |
History
A 67-year-old black female reported to the office with a chief complaint of painful eyes, more so in the left eye than the right, for the previous week.
She explained she was experiencing pain, redness and photophobia in both of her eyes, but the discomfort in the left eye had become unbearable.
Her systemic history was remarkable for hypertension, which was properly medicated. She denied allergies of any kind.
Diagnostic Data
Her best corrected entering visual acuities were 20/20 OD and 20/20 OS at distance.
We performed an external examination and the results were normal with no evidence of afferent pupil defect. The biomicroscopic examination of the anterior segment of both of her eyes showed no iris neovascularization.
The pertinent anterior segment findings include the cell flare in the left eye (Figure 1). Goldmann applanation tonometry measured 15mm Hg OU.
The pertinent clinical findings in the posterior segment of the patient’s left eye are demonstrated in Figure 2.
Your Diagnosis
Does this case require any additional tests? How would you manage this patient? What is the likely prognosis? What does this patient’s history and clinical findings tell you about her most likely diagnosis?
Discussion
Additional studies included epilation of the misdirected eylashes, fluorescein staining to ensure there was no corneal epithelial defect, indirect stereo biomicroscopic examination of the fundus, three–mirror lens evaluation of the prescribed area along with scleral depression. Photodocumentation was also completed.
The diagnosis in this issue is dual: traumatic anterior iritis secondary to trichiasis in the left eye and superior vitreous-tractional retinal flap tear, also in the left eye.
The offending lashes were epilated in both eyes, and the patient’s left eye symptoms were addressed with cycloplegia prednisolone acetate 1% QID, topical lubrication and oral over-the-counter analgesics. To complete the inspection of the ocular tissues, while therapeutic cycloplegia was instilled in the left eye, both eyes were dilated. There was lattice degeneration seen in both eyes, with a clear retinal break with subretinal fluid superiorly in the left. The shallow retinal detachment was confirmed by a retinal specialist the same day and was treated with a barrier laser. A follow up was scheduled for two months and will be scheduled at six month intervals there after. She was educated regarding her trichiasis and will be monitored regularly at three to four month intervals or as needed.
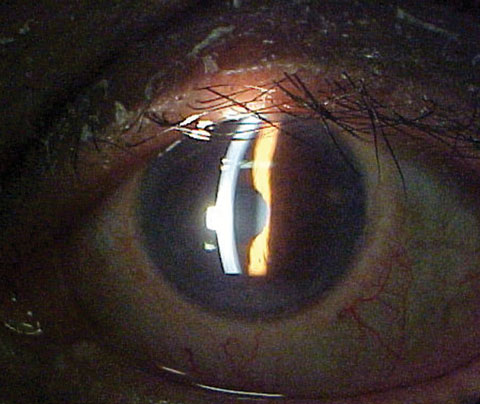 |
| Fig. 1. The anterior segment of our 67-year-old patient’s left eye. |
Tractional retinal tears (TRT) may lead to rhegmatogenous retinal detachment (RRD).1-25 They have no specific racial, gender or laterality predilection; rather are a phenomeneon produced by complications of other pathologies which induce preretinal tension.1-32 The phenomenon of retinal tears is more common in the older population. TRT is associated with pathological myopia with staphylomatous retinal stretching and vitreoretinal tension created by vitreoretinal interface abnormalities such as white with and without pressure, degenerative retinoschisis, cystic retinal tufts, epiretinal membrane, vitreomacular traction syndrome and, rarely, zonular traction tufts on or near the border of lattice degeneration.7-10,13-16,32 Other vitreoretinal pathologies including Wagner’s syndrome and Stickler’s syndrome increase the risk of TRT.19 While intraretinal neovascular diseases, such as proliferative diabetic retinopathy, ischemic venous occlusion and proliferative sickle cell retinopathy, can induce fibrovascular traction, which create TRT and tractional retinal detachment (TRD), the typical cause is robust interaction of the vitreous along the border of vulnerable retina.1-30 In one study the incidence of TRT in eyes with a symptomatic posterior vitreous detachment (PVD) was 8.2%.31 TRT are also associated with systemic diseases such as Marfan’s syndrome, Ehlers-Danlos syndrome and homocystinuria.19
TRT often assumes one of three forms: the flap tear (horseshoe-shaped tear), a retinal tear adjacent to an area of lattice degeneration or an operculated tear.1,2,4,14-19 The incidence of RRD without retinal breaks in the general phakic population is generally low (12:100,000).1-4,7,8,19 The natural prevalence of RRD increases with myopia from 4.3% in emmetropia to 14% in myopia of greater than −3 D, however, the risk increases dramatically when a symptomatic TRT is present.18,20 Although one study suggests the incidence of RRD from retinal breaks associated with lattice degeneration is low (0.3% to 0.5%), lattice degeneration may be associated with up to 60% of RRD.18
Patients with TRT often report a sudden onset of either a single or multiple floating spots, along with flashing lights (photopsiae).10-12 Unlike entoptic phenomena which demonstrate exacerbations and remissions or the scintillating scotoma produced in vasospastic events, the visual symptoms remain stable in the patient's visual field.10-12 Pain is not a feature of any retinal detachment as the tissue has no pain receptors. There may be precipitating ocular or head trauma. If there has been a vitreous hemorrhage, there will be multiple large floaters or opacities which may take the form of “cobwebs.”10-12 There may be severe loss of vision if dense vitreous hemorrhage interrupts the visual axis or if a resultant RRD or TRD involves the macula. It is also possible the patient is asymptomatic and unaware anything has occurred.12 The blood or retinal pigment epithelium (RPE) debris released from a TRT can be observed by the clinician during fundus examination and should be noted as “tobacco dust” or “Schaffer’s sign.”29
Retinal breaks are defined as full thickness defects in the neurosensory retina.1,2,7-10,11,19 Although they typically occur in the equatorial region posterior breaks are plausible. All retinal detachments involve a dissection of the neurosensory retina from its underlying RPE layer by subretinal fluid (SRF).4,5 The principle involved in TRT and RRD is that forces exerted by the vitreous or neovascularization at the site of their attachment to the retina overcome its tensile strength, creating a full thickness discontinuity through which fluid can migrate, prying the neurosensory retina from the underlying RPE.3,5,13,14,19
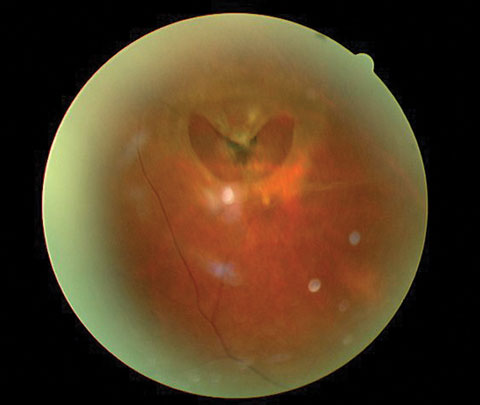 |
| Fig. 2. The posterior segment of the same eye. The patient had experienced pain for approximately one week. |
The most common natural inciting processes creating a TRT is posterior vitreous detachment.19-24 Epiretinal membrane formation and vireomacular traction syndrome are also commonly associated with TRT.20-25 The vitreous humor is formed by a meshwork of collagen fibrils that provide a scaffold-like structure formed by hyaluronic acid.10 Firm attachments of the vitreous to the retina occur via chemical bonds through laminin, fibronectin and sulfated proteoglycans.10 The areas of firm adhesion include the vitreous base, the margin of the optic disc, the back of the crystalline lens (hyloidocapsular ligament of Wieger), the fovea, along large retinal vessels and sites of abnormal vitreoretinal anatomy such as the margins surrounding lattice degeneration.10,13,32,33 PVD begins with synchysis (vitreous liquification). This weakens the vitreoretinal adhesions.10 As the vitreoretinal adhesions dissolve, discontinuities form within the posterior hyaloid (either via fissure evolution or a microbreak in the thin cortical vitreous layer).22 This allows liquid vitreous to enter the subhyaloid space dissecting the posterior hyaloid from the ILM.1,11,20-23 An anomalous PVD (APVD) results when synchysis occurs without complete detachment from the ILM.27 This results in tractional effects at the interface.1,1120-23,32,33 The physics of anomalous PVD has the potential to generate forces which split the posterior vitreous cortex causing vitreoschesis.40 When this phenomenon occurs, tractional forces increase the risk of TRT and RRD, especially at the margins of anatomically thin retina (lattice degeneration).1,13,32,33 One classic study identifies the pathogenic principles of TRT to include association with three topographical relationships to the vitreous base: intrabasal-caused by avulsion of zonular traction tufts; juxtabasal-related to traction of the posteriorly detached vitreous on irregularities in posterior border of vitreous base and extrabasal-resulting from avulsion of cystic retinal tufts.32
When these forces project adjacent to the macula, they have the potential to induce wrinkling of the neurosensory retina referred to as macular pucker.1,13 The pathology is also known as cellophane maculopathy, epiretinal membrane, vitreoretinal interface maculopathy and vitreo-macular traction syndrome (VMTS).1,13,24 Over time, as traction increases in either force or size, it can create a dehiscence (TRT/break) that initiates the formation of macular hole.1,13,24 Hyperconvolution and duplication of the ILM along with the proliferation of preretinal glial membranes (epiretinal membrane) are the distinctive features which create the tractional forces on the retina, increasing the risk of TRT.20,22
Traumatic retinal breaks are theorized to result from rapid globe distortion with expansion and contraction. Here, vitreoretinal traction at the orra serrata and equator induces TRT, irregular breaks or retinal dialysis (circumfrential breaks).19,26,27 Traumatic retinal breaks are documented to have an increased incidence in the inferotemporal and superonasal regions.19,27,28
Horseshoe-shaped tractional tears are triangular in appearance with a u-shaped flap inside a u-shaped tear. The apex of the tear (posterior edge) remains attached to mobile vitreous and points towards the posterior pole.1,19 The base of the tear is anchored at the vitreous base. Identical pathophysiology permits mobile vitreous to potentially enlarge the tear and physically separate it from the RPE creating “tobacco dust.”1,19,30 If the tear bridges a blood vessel, there can be subsequent vitreous hemorrhage.16 If an area of retinal tissue is pulled completely free and is observed to be floating in the vitreous, the lesion is considered an operculated tear with the free retinal tissue, termed the operculum.19 Operculated tears often signal complete vitreoretinal traction release.19 Classic evidence suggests that the edges of retinal breaks are covered by smooth cellular membranes, merging peripherally with a meshwork of vitreous fibrils.16 These membrane cells have poorly defined borders, a pitted surface and a variable number of microvilli.16 Lattice surfaces and paravascular retinal degenerations seem to be covered by similar membranes with subtle microscopic differences.16 It appears that breaks in the internal limiting membrane always stimulates the proliferation of preretinal glial membranes.16 Limited proliferation of these membranes suggests that surface gliosis is normally inhibited when the cells contact either intact basement membrane or vitreous.16
The management of a TRT (observation vs. protective intervention) depends upon weather the risks of treatment outweigh the risks of retinal detachment.1,2,15,19,30 Tear location (superotemporal), size (larger TRT increase risk), symptoms (number one consideration), history of retinal detachment in the same eye or fellow eye, history of retinal detachment in the family, lifestyle (active vs. sedentary), the presence of myopia (greater than 6 diopters), the patient’s phakic status (phakic, pseudophakia, aphakia) and the patient’s potential for undergoing cataract surgery are all important factors.1,2,15,19,30 One study reported the rate of retinal detachment in symptomatic phakic patients with TRT to be 35%.19,34 The study strongly recommended proactive treatment.19,34 The literature continues to support “symptoms” as the most important criterion for considering intervention.15,19 The modalities used to create the protective barrier around TRT preventing sub-retinal fluid (SRF) infiltration and subsequent RRD are generically known as retinopexy (cryopexy and barrier laser photocoagulation).19,30 Cryotherapy (cryopexy-transconjunctival cold application) creates a seal between the chorioretinal tissues by destroying choriocapillaris, RPE and outer retinal elements.19,30-35 The procedure requires three weeks for the seal to mature mandating reevaluation in that time period.19,30-33 Barrier laser photocoagulation uses argon blue-green, krypton red or diode delivery systems to create the same effect. Evidence suggests significant effects occur immediately with the maximal effect occurring in 10 days.19,30-35
Evidence suggests that cases of TRT exhibiting vitreous hemorrhage should be considered for early vitrectomy.38 While cataractogensis is an undisputed complication of vitrectomy, the benefit of increased visual acuity and increased ability to find and assess the TRT and the possibility of RRD seems to outweigh that complication .38
Macular holes and lost acuity created by vitreomacular traction syndrome are traditionally released using three-port pars plana vitrectomy.34 If an epiretinal membrane is present it too may require peeling.22,37 A novel pharmaceutical approach has been developed using an intravitreal injection of the recombinant protease agent microplasmin such as Jetrea (Ocriplasmin, ThromboGenics) which targets fibronectin and laminin at the vitreoretinal interface.36 The agent has shown promise for releasing vitreous traction (25% published and anecdotally) and closing macular holes (40% published and anecdotally).38
- Tractional tears without symptoms or significant risk factors may be safely monitored without treatment, however, a retinology consult is advised to confirm this strategy.
- The RPE often becomes hyperplastic following an insult that produces a retinal tear. This chorioretinal scar may act as a natural seal around the break. When this happens there is a lower risk of tractional/rhegmatogenous detachment.
- Since eyes with a symptomatic posterior vitreous detachment develop retinal breaks in 8.2% of cases, patients presenting with new onset PVD with symptoms should be advised to limit strenuous activity and contact sports for a period of three to six weeks following the event. These patients should be redilated and examined within two to five weeks of the initial presentation to confirm stable anatomy.
- In all surgical cases, topical cycloplegic, antibiotic and steroidal drops are commonly prescribed post operatively. Follow up should include vision, pressure and retinal examination.
- Asymptomatic atrophic retinal holes in lattice rarely lead to RRD and do not need prophylactic treatment. If SRF is present or if scleral depression/three-mirror lens examination cannot be accomplished an expert opinion should be sought.
|

