As practitioners who care for glaucoma patients, we have the responsibility to recognize when our patients require advanced care to prevent progressive vision loss from this blinding disease. When glaucomatous progression continues after treatment with medications and laser procedures, advanced surgical intervention is often necessary.
Historically, penetrating filtering surgeries—namely trabeculectomy and aqueous tube/shunt implantation—have produced significant and sustained IOP reductions to halt the progression of optic nerve damage and visual field loss. However, they sometimes carry significant risks.
In recent years, however, study has focused on many new “non-penetrating” procedures that don’t create a full thickness sclerostomy. As a group, these non-penetrating procedures are thought to be more challenging technically, but have fewer and less severe short-term complications than trabeculectomy.1 While the improved safety is counterbalanced by not achieving a lower IOP than trabeculectomy, over the last decade, the number of trabeculectomy procedures has declined while the use of some of the newer procedures highlighted below are on the rise.1,2
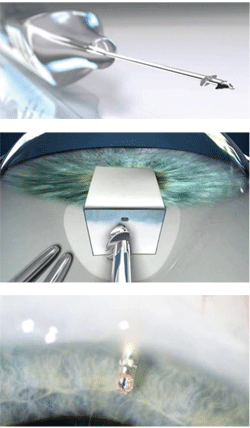
1-3. The Ex-PRESS tube shunt procedure.
Courtesy: Alcon Laboratories
Filtering Surgery Alternatives
In some high-risk cases and in cases involving repeat surgery, tube shunts have distinct advantages. One of the newest filtering devices, the Ex-PRESS tube shunt, is intended to be used in more widespread situations as an alternative to traditional trabeculectomy. This FDA-approved device is thought to combine the benefits of previous shunts while being easier to use with even fewer complications. The Ex-PRESS device is made of stainless steel, measures less than 3mm in size and comes preloaded on a handheld insertion device (figure 1). It is implanted under a scleral flap to drain aqueous from the anterior chamber into the subconjunctival space as a trabeculectomy does (figure 2). Likewise, it creates a conjunctival bleb, but with fewer complications than a traditional trabeculectomy. First, a peripheral iridectomy (PI) is not required because the tube is implanted well into the anterior chamber to avoid iris tissue blocking the Ex-PRESS lumen (figure 3). Lack of a PI helps to control risks of hyphema and postoperative inflammation. Moreover, the controlled flow provided by the constant lumen size of the device provides greater safety against postoperative hypotony. This controlled aqueous flow can also limit the IOP-lowering effect of the surgery. One study noted that in comparison to trabeculectomy early postoperative IOP measured higher, but by three months, IOP reductions were similar to trabeculectomy.3 The early promising results have resulted in the Ex-PRESS device’s accounting for about half of all aqueous shunt procedures performed today.2
TM Outflow Enhancement
Unlike a trabeculectomy or a tube procedure that purposefully bypasses the normal aqueous drainage system, many new procedures and devices specifically target the physiological outflow tissues and try to enhance the drainage through the existing structures. The Trabectome is an FDA-approved procedure involving a microelectrocautery instrument that ablates a portion of the trabecular meshwork (TM), juxtacanalicular tissue and Schlemm’s canal to reduce resistance to outflow through these existing drainage tissues.4 The procedure has also been called “Ab interno trabeculotomy” because the device approaches Schlemm’s canal internally from the anterior chamber through a clear corneal incision while viewing the device interacting with the meshwork through gonioscopy (figures 4 and 5).
A 60° to 120° electrocautery incision is performed directly into the TM.4 Therefore, ideal candidates should have an easily viewable open angle with no or minimal peripheral anterior synechiae. Since the entry point for this procedure is through the anterior chamber, it can be done in combination with cataract surgery.
A retrospective review of 1127 cases treated with Trabectome surgery showed it to be a safe and effective alternative to filtering surgery.5 Those having the Trabectome-only procedure (without cataract surgery) reported a mean decrease in IOP of 32% at 60 months (mean pre-op IOP of 25.7mm Hg vs. mean post-op IOP of 16.4mm Hg).5 Additionally, postoperative use of glaucoma medications decreased by 39%.5
This procedure is largely effective at lowering IOP because it targets the TM and inner wall of Schlemm’s canal that has long been thought to be the source of the greatest resistance to outflow.4 The limtation of the Trabectome procedure is that IOP does not reach single digits since it is still limited by episcleral venous pressure.4 However, complications with are infrequent with the most common being an IOP spike exceeding 10mm Hg above baseline at day-one in 5.8% of cases.5
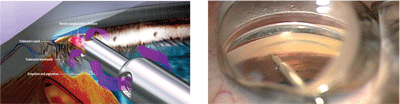
4, 5. A Trabectome procedure enhances drainage through existing structures. Courtesy: NeoMedix Corporation
Another device, the iStent, is an implantable device that is also aimed at aiding normal aqueous drainage into Schlemm’s canal (figure 6). It is a small (120µm diameter) titanium device that comes on a preloaded applicator that is implanted into the anterior chamber angle via an internal approach similar to that of the Trabectome (figure 7). It has been described as a micro-bypass system in that it helps to bypass the resistance of the TM and juxtacanalicular tissue by establishing flow directly into Schlemm’s canal.6
While this device is not yet FDA approved, initial results have been promising. An initial report showed that iStent in combination with cataract surgery resulted in 62% of patients achieving IOP of ≤18mm Hg post-op without the use of medication.7 Additionally, there were no reports of hypotony, flat chambers or reduction in VA at 12 months post-op.7
Like the Trabectome, postoperative IOP with the iStent will still be limited by episcleral venous pressure. Nevertheless, because not all patients need to have a single digit IOP, these procedures are often done before a trabeculectomy. Because neither procedure results in bleb formation, the conjunctival tissue is preserved in case a future trabeculectomy is needed. In addition to avoiding a bleb, neither the Trabectome nor iStent surgery requires a PI, which also helps to reduce postoperative side effects.
Viscocanalostomy and Canaloplasty
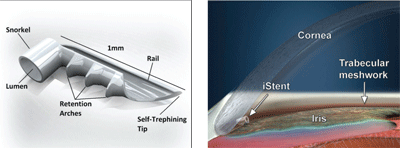
6, 7. The iStent is an implantable device aimed at aiding normal aqueous drainage into Schlemm’s canal. Courtesy: Glaukos Corporation.
Like the Trabectome procedure, viscocanalostomy and canaloplasty procedures target the tissues of the normal physiologic aqueous drainage—specifically the inner wall of Schlemm’s canal. However, as opposed to entering the canal from an internal approach via the anterior chamber, the canal procedures involve entering the canal from an external approach through a surgical scleral flap. For lack of a better analogy, the viscocanalostomy procedure has been compared to “roto-rootering” Schlemm’s canal.
After creation of the scleral flap and exposure of Descemet’s window, an injection of viscous solution is used to dilate and irrigate Schlemm’s canal with the intent of reducing the resistance to outflow within this structure. Unlike a trabeculectomy, it has the benefit of not intentionally creating a conjunctival bleb because it is aimed to enhance the normal aqueous flow ending in the episcleral venous system. This reduces risks and complications, but also reduces the IOP-lowering efficacy of the procedure.
A comparison study of viscocanalostomy versus trabeculectomy showed a mean postoperative IOP of 16.88mm Hg for the viscocanalostomy group compared to 14mm Hg for the trabeculectomy group.8 Interestingly, this study showed a higher rate of cataract formation in the viscocanalostomy group, which was unexpected because trabeculectomys are known to be cataractogenic.8 Another unexpected complication of viscocanalostomy was spontaneous bleb formation, postulated to be from aqueous drainage exiting the surgical entry point where Schlemm’s canal was cut.8 However, the relative safety of viscocanalostomy showed fewer complications of hypotony, wound leak and choroidal detachment.8
A canaloplasty, on the other hand, takes the concept of altering Schlemm’s canal one step further by not only dilating the canal, but also implanting and tightening a suture within the inner wall of the canal to reduce outflow resistance and promote even further drainage. Like viscocanalostomy, a canaloplasty starts by exposing Schlemm’s canal through the creation of a scleral flap. Then, a microcatheter is advanced circumferentially to dilate the entire 360° of the canal. Once dilation is complete, a suture is tied to the catheter. As the catheter is withdrawn from the canal, the suture is threaded into position throughout the circumference of the canal. The suture is tied off to create tension on the inner wall of the canal.
There is some debate as to how tight to tie this suture and how the degree of tension might affect the outflow. The suture tension itself causes increased permeability of the inner wall. However, putting too much tension on the suture can cause it to “cheese wire” or puncture through the inner wall of the canal. Leaving too little tension may impair the IOP lowering efficacy of the procedure. Additionally, one author speculates that there may be variability in the amount of tension needed from individual to individual.9
One study of canaloplasty as the initial surgical intervention on medically uncontrolled glaucoma patients showed a consistent drop in postoperative IOP. Mean preoperative pressures of 27.3±5.6mm Hg dropped to 12.8±1.5mm Hg at 12 months and 13.1±1.2mm Hg at 18 months postoperatively.9 This allowed patients to be maintained on significantly fewer medications following canaloplasty. The number of preoperative medications (2.7±0.5) dropped to an average of just 0.1±0.3 medications postoperatively.9 Additionally, a comparison study of canaloplasty and viscocanalostomy showed that canaloplasty was more likely to reach an IOP ≤17mm Hg, doing so 83% of the time compared to just 42% for viscocanalostomy.9
Uveoscleral Outflow Enhancer (Suprachoroidal Shunts)
The uveoscleral outflow pathway is well known due to the widespread use of prostaglandins as initial medical therapy for glaucoma. However, the complex mechanism of this pathway is just now becoming well understood. It appears that the resistance to uveoscleral outflow is primarily in the uveal portion as aqueous moves through the longitudinal ciliary muscles to reach the suprachoroidal space.10 From there, the aqueous fairly easily exits by diffusing through the sclera and draining into the orbital lymphatic system.10 This has led to the development of devices that can drain aqueous past the high resistance area of the longitudinal muscles directly into the suprachoroidal space.
Unlike the iStent device that is inserted directly into the angle structures via an anterior chamber approach, these devices use an external approach and require conjunctival resection and a scleral incision to expose the supraciliary space. However, like the other procedures described above, suprachoroidal shunts do not require a peripheral iridectomy and avoid the creation of a conjunctival bleb. Similarly, this has the advantage of reducing complications of hypotony and infection while preserving the integrity of the conjunctival tissue should a future bleb procedure be necessary.
The gold microshunt by SOLX is suprachoroidal shunt that is approved for use in Canada, but is still under investigation by the FDA. A small study testing the effectiveness of the gold microshunt showed a decrease in mean IOP from 27.6mm Hg to 18.2mm Hg without any complications of hypotony or infection.11
Interestingly, this device has multiple channels built into the implant. At the time of implantation, only some of these channels are patent with the possibility to open up more channels noninvasively with a laser if greater effect is needed postoperatively.
An even newer device in this category is the Aquashunt, developed by Dr. Bruce Shields of Yale University and marketed by Opko. Unlike the gold microshunt, this device has one large channel to allow for the possibility of lower IOP results. The Aquashunt is not yet FDA approved and is undergoing clinical testing at the time of this writing.
Aqueous Suppression: Endocyclophotocoagulation
Cyclodestruction of the ciliary body has traditionally been a procedure reserved for advanced cases of “end stage” glaucoma to help maintain comfort to a badly damaged eye suffering from uncontrolled IOP. This procedure was limited to these severe cases due to side effects of the procedure itself when the photocoagulation was done transsclerally with an external probe. When performing cyclodestruction transsclerally, there is significant discomfort and scarring of adjacent structures since the ciliary body processes cannot be targeted directly.
This often led to overtreatment and hypotony.
A newer approach allows the direct ablation of the ciliary body endoscopically with the probe inserted through a corneal incision and advanced through the anterior chamber to the ciliary processes in the posterior chamber.
Because this is a penetrating procedure that exposes the eye to risk of infection, it is commonly combined with cataract surgery when that risk is going to be incurred anyway. The cataract procedure itself is conducted no differently followed by endocyclophotocoagulation (ECP) using the same phaco port after phacoemulsification and IOL implantation has taken place. The ECP can ablate 180° to 360° of tissue, but one author suggests treating at least 270°.12
When combining ECP with cataract surgery there is always a question as to how much of an additional effect ECP provides over cataract surgery alone. A prospective study of 707 eyes that followed patients postoperatively for 3.2 years, showed that the phaco/ECP group showed an IOP reduction to 15.73mm Hg from a preoperative mean IOP of 19.08mm Hg while the phaco alone group showed no net reduction in IOP.13 Additionally, the phaco/ECP group reduced their medications from an average of 1.53 preoperatively to 0.65 postoperatively, while the phaco-alone group experienced no reduction in medication use.13
So, when is ECP the right choice? ECP is typically recommended for a glaucoma patient who is well controlled on medications undergoing cataract surgery. Because a large IOP drop is unexpected, it is often the goal to reduce the number of medications in a fairly controlled glaucoma patient instead of trying to treat an uncontrolled pressure of an advanced glaucoma patient.12
Because optometrists may be involved in the postoperative management in patients with combined phaco/ECP procedures, they should be aware of key points in caring for these patients. A colleague of mine has noted that while postoperative medications are typically the same as traditional cataract regimens (a topical antibiotic, NSAID and steroid), steroid dosing starts more aggressively and lasts longer than a typical phaco-only procedure. Topical prednisolone acetate dosing starts at six times daily for one week to combat postoperative inflammatory IOP spikes. Additionally, her office utilizes acetazolamide 250mg p.o. b.i.d. for two to three days for additional postoperative IOP control. Topical NSAID use is extended to four weeks postoperatively to reduce the risk of CME development.
Clearly, the landscape of glaucoma surgical care is vast and growing. The surgical advancements described here are just a sample of many new and exciting procedures that can offer patients options for gaining reductions in IOP with fewer complications.
Penetrating filtering procedures like trabeculectomy and traditional tube shunts are still the surgical treatment of choice for advanced glaucoma cases requiring a single digit IOP.
However, optometrists should be aware of all the options that are available for their patients and be able to recognize the
appropriate patients to refer for a surgical consult. Many of these individuals might be medically controlled glaucoma patients in need of cataract surgery who may be able to combine a glaucoma procedure with phacoemulsification to reduce their dependence of glaucoma medications.
Additionally, optometrists should be able to critically evaluate their patients to recognize the clinical signs that a patient may have already undergone one of these new procedures. n
Dr. Nixon is an associate professor of clinical optometry and director of extern programs at The Ohio State University College of Optometry. He is also in a group private practice in Westerville, Ohio. He has no financial interest in any of the companies or products mentioned in this article.
1. Weinreb RN, Crowston JG. World Glaucoma Association Consensus Series 2: Glaucoma Surgery. Kugler Publications, 2005. ISBN: 978-90-6299-203-4.
2. Corcoran KJ. The Shifting Tides of Glaucoma Surgery. Rev Ophthalmol. 2009;16:09.
3. Maris PJG, Ishida K, Netland PA. Comparison of Trabeculectomy with Ex-PRESS miniature Glaucoma Device Implanted Under Scleral Flap. J Glaucoma. 2007 Jan; 16:14-9.
4. Francis B. Update: Lowering IOP With the Trabectome. Rev Ophthalmol. 2007;14:4.
5. Minckler D, Mosaed S, Dustin L, et al. Trabectome (trabeculectomy-internal approach): Additional experience and extended follow-up. Trans Am Ophthalmol Soc. 2008;106:149-60.
6. Nichamin LD. Glaukos iStent® Trabecular Micro-Bypass. Middle East Afr J Ophthalmol. 2009 Jul-Sept;16(3):138-40.
7. Spiegel D, Wetzel W, Neuhann T, et al. Coexistent primary open-angle glaucoma and cataract: interim analysis of a trabecular micro-bypass stent and concurrent cataract surgery. Eur J Ophthalmol. 2009;19:393-9.
8. Gilmour DF, Manners TD, Devonport H, er al. Viscocanalostomy versus trabeculectomy for primary open-angle glaucoma: 4-year prospective randomized clinical trial. Eye. 2009:23(9):1802-7.
9. Grieshaber MC, Fraenkl S, Schoetzau A, et al. Circumferential viscocanalostomy and suture canal distension (canaloplasty) for whites with open-angle glaucoma. J Glaucoma. In press.
10. Kent C. Uveoscleral Outflow: A Better Way to Go? Rev Ophthalmol. 2010;17:3.
11. Melamed S, Simon GJB, Goldenfeld M, Simon G. Efficacy and safety of gold micro shunt implantation to the supraciliary space in patients with glaucoma. Arch Ophthalmol. 2009;127(3):264-9.
12. Lin S. Making the most of ECP and TCP. Rev Ophthalmol. 2007;14:6.
13. Berke SJ. Data Supports Safety and Efficacy of Phaco/ECP. Rev Ophthalmol. 2006;13:6.

