Epiretinal membrane (ERM)––also known as macular pucker, vitreoretinal interface maculopathy and cellophane maculopathy––results when a thin formation of fibrous tissue develops on the surface of the retina. Potential causes of ERM include trauma, retinal detachment, inflammatory disease and vascular disease.1-5 In most instances ERM development is idiopathic and age-related.1,2 When ERM evolves in the macular region, it can cause a host of ocular sequellae, ranging from an asymptomatic presence to reduced visual acuity and metamorphopsia.1-5
This unique pathophysiological phenomenon develops secondary to cellular changes, which co-mingle between the posterior vitreous and internal limiting membrane (ILM) of the neurosensory retina.1-5 It is important to note, however, that ERM is the most common precursor to vitreomacular traction (VMT) syndrome.1,2
Here, we examine a patient who presented with VMT syndrome. In addition, we review the condition’s pathophysiology as well as discuss several established and novel treatment options.
Case Report
• History. A 78-year-old black female presented with a chief complaint of visual acuity loss in her right eye that had persisted for the past 10 days. Her ocular history was significant for ERM, advanced optic nerve cupping and an optic pit in her right eye. She also had a long-standing history of stable 20/30 visual acuity O.U.
She denied any new trauma and described the central vision in her right eye as “smudged.” Her systemic history was unremarkable. She took no medications and denied having allergies of any kind.
• Diagnostic data. Her best-corrected entering visual acuity measured 20/200 O.D. and 20/30 O.S., with no improvement upon pinhole testing. We detected no afferent defect, change in color vision or alteration in comparative brightness sense. Confrontational visual fields uncovered a blurry, distorted, central field, which was confirmed with formal Amsler grid testing. Retinoscopy demonstrated a clear reflex without distortion, and refraction found negligible changes to the current spectacle prescription (which measured +0.50D O.U.).
Biomicroscopy revealed normal and healthy anterior segment structures; mild, but symmetrical, nuclear lenticular opacities; open angles; and normal intraocular pressure, which measured 16mm Hg O.U. Dilated fundus examination exhibited premacular fibrosis with macular traction O.D., which was confirmed on spectral domain optical coherence tomography (SD-OCT).
• Discussion. The diagnosis in this case was VMT syndrome in the patient’s right eye. We referred her to a retinal specialist to confirm the diagnosis and rule out a surgical solution. The retinal specialist informed our patient of the surgical procedures necessary to potentially improve her vision; however, she declined to proceed. We scheduled our patient for a four-month follow-up with the general ophthalmic service as well as a six-month follow-up with the retina service.
Pertinent Anatomy
Light is comprised of photons that enter the eye through the pupil and penetrate the ocular media (cornea, aqueous, lens, vitreous) and neurosensory retina. The photons then reflect off the retinal pigment epithelium (RPE) to depolarize the photoreceptors (rods and cones).
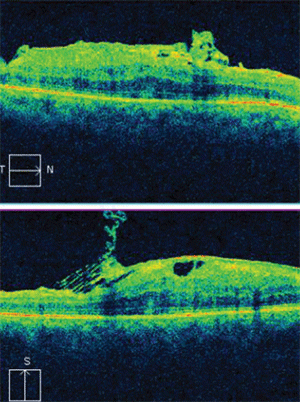
Horizontal and vertical SD-OCT scans of our patient’s right eye revealed tractional elevation of the retina at the macula.
With the assistance of horizontal, bipolar, amacrine and retinal ganglion cells, light reaches the occipital lobe of brain via the visual pathway. This process results in vision. Supporting glial cells and Müller cells interdigitate with this neuronal pathway, enabling the necessary structural architecture.2,6-10
Any disruption that causes the retina to inefficiently receive light, or any discontinuity in the pathway from the neurosensory retina to the brain, can cause visual disability. This includes refractive error; corneal disease; anterior chamber phenomena, such as cells and protein from uveitis; blood from hyphema; lenticular opacity; or vitreous abnormality, such as vitreous cells, hemorrhage or retinal traction.1-12
The vitreous is a clear, gelatinous matrix that is largely composed of water. Collagen fibers and hyaluronate particles bind the water, creating a support structure for the vitreous body.13-15 Condensed collagen fibrils that form the vitreous cortex superficially insert into the ILM of the retina or basal lamina of the Müller cells. The vitreous is firmly attached before the equator, just posterior to the ora serrata, at the vitreous base. This circular band is approximately 4mm wide and serves as the strongest of all vitreal attachments. Secure attachments are also present at all major retinal vessels, the area of Martgiani around the optic nerve and the center of the macula.13-15 At the macula, the vitreous is attached in concentric zones that center on the 500µm-diameter foveola.14,15
Epidemiology
VMT syndrome is defined as macular traction associated with a partial posterior vitreous detachment.12,16-18 A complete posterior vitreous detachment (PVD) involves a separation of the posterior hyaloid from the ILM, and is not a risk factor for VMT syndrome. The prevalence of PVD increases with age. Vitreous syneresis and synchysis, intraocular surgery, high myopia and trauma may contribute to PVD development.12,16-18
Sixty-eight percent of patients over age 65 have PVD.18-20 There is no racial predilection for ERM or VMT syndrome.18-20 Multiple studies have indicated that 60% to 70% of PVD and VMT syndrome cases occur in women.18-20 Because a PVD is a common consequence of intraocular surgery, pseudophakic patients are at a decreased risk of VMT syndrome. Additionally, the majority of nontraumatic VMT syndrome cases occur in patients aged 60 to 80 years.1,2,5,18,19
The course of VMT syndrome is variable in presentation. Patients may show a gradual or abrupt decrease in vision. Visual acuity may remain stable or even improve following a complete spontaneous PVD.21,22 Patient improvement is verified by direct observation of decreased symptomology and/or medical imaging. Further treatment is necessary when the condition threatens visual acuity. Initial visual acuity, symptom duration and associated macular complications, such as cystoid macular edema, play a role in the postoperative prognosis of VMT syndrome.21,23
Pathophysiology
With increased age, the collagen-hyaluronic acid mixture of the vitreous humor liquefies and shrinks. Additionally, the ILM thickens. The combination of vitreal and retinal changes results in decreased vitreoretinal adhesion throughout the fundus.11,24 The weakened fundus promotes vitreous migration into the subhyaloid space. Typically, the vitreous displaces the posterior surface of the hyaloid from the ILM, causing a complete PVD. In the case of VMT syndrome, however, a complete separation of the vitreous and retina does not occur, creating tractional forces at the level of the macula.2,5,11,17
VMT syndrome leads to decreased vision, metamorphopsia, microsia and/or photopsia. Macular traction secondary to the incomplete PVD creates distortion and/or retinal striae at the macula. This distortion can affect any macular cellular layer, including surrounding blood vessels.12,18 Any disturbance among the tight junctions of retinal vessels (or the zonula occludens junctions) associated with the RPE can permit fluid movement, which can cause edema and thickening of the surrounding neurosensory tissue.12,14,15
An ERM represents glial cell proliferation between the ILM of the neurosensory retina and the posterior hyaloid membrane of the vitreous humor.11,12 ERM may result from a variety of etiologies, including trauma, inflammation and vascular damage, or may arise idiopathically. Growth, spreading and thickening of ERM ultimately can lead to VMT syndrome.1-3,11,24 Weakness in the retina and/or increased tensile force on the retina can lead to rhegmatogenous tears anywhere that traction exists.13,15,25
Fibrous astrocytes are the primary cell type involved in VMT syndrome. Fibrocytes and myofibrocytes, as well as cellular fragments of the internal limiting membrane, collagen and macrophages, may play a vital role in the development of VMT syndrome.1,2
Two main pathophysiological aspects of epiretinal fibrocellular proliferation can result in VMT syndrome. The two processes are distinguished by the presence of cortical vitreous following separation of the posterior vitreous.5,12,17,18 When cortical vitreous is present, the attachment causes a cystic edema of the macula with a resultant decrease in vision.12,20 As the pathophysiological outcome of the ERM evolves, interposed vitreous collagen is remodeled when single cells or a cellular monolayer grows directly onto the ILM. It is also important to note that VMT syndrome is a potential precursor for macular hole formation.12,16,17,26,27
Both pathophysiological forms of VMT syndrome have shown spontaneous regression following preretinal membrane peel with complete detachment of the posterior vitreous in the macular region. Such changes eliminate the visually debilitating architecture and associated symptoms as well as any need for further intervention.5,16,17,26,27
Conventional Management
Detection of ERM and VMT syndrome is best accomplished with stereoscopic indirect biomicroscopy using a 60D, 78D or 90D lens or on contact 3-mirror direct funduscopic evaluation. On funduscopic evaluation, ERM appears as a shinny, yellow, reflective area located either on top of or interspersed within the retina.
Advanced imaging systems, including OCT, have greatly increased recognition of VMT syndrome.21,23,28 OCT imaging illustrates VMT syndrome as a minimally reflective band above the retina with a focal area of detachment in the presence of central adhesion to the fovea.23,24,28 When ERM or VMT syndrome is suspected secondary to signs or symptoms, OCT imaging readily permits the differential diagnosis as well as helps identify the presence of macular hole formation.25,29,30
Treatment of VMT syndrome includes careful monitoring with fundus examination and serial OCT at four- to six-month intervals. Surgical intervention is only required when the visual disturbance reduces quality of life. In cases that require surgery, vitreofoveal traction may be relieved via 3-port pars plana vitrectomy (PPV) with posterior hyaloid membrane peel.25,29,30
If the patient also exhibits a macular hole, 3-port PPV is completed with intraocular gas tamponade and then the individual is placed face-down.5,18,30 Studies indicate a visual improvement with anatomical restoration after PPV in 50% to 75% of cases.18,20 ILM peel may improve anatomical outcomes, but often yields a less predictable visual outcome. After surgery, OCT may be used to monitor outcome control and help predict the prognosis.25,29,30
New Treatment Options
Currently, the use of enzymatic vitreolysis is being investigated as an alternative to vitrectomy surgery.31,32 The Microplasmin for Intravitreous Injection-Phase II Trial (MIVI-IIT) evaluated the ability of either a single or repeated injection of microplasmin (ThromboGenics) to release VMT.31 One report indicated that 125µg injections of microplamin successfully released vitreoretinal traction when compared to a placebo injection.32
The use of robotics also may be a valuable tool in the surgical treatment of VMT syndrome. Precision, repeatability and automation reduces the risk of human error. Robotic procedures can also eliminate the need for vital dyes, which are often employed by surgeons for better visualization during membrane peel procedures.33
The Da Vinci surgical system (Intuitive Surgical) is an FDA-approved robotic prototype for human surgery.34-37 The unit consists of three robotic arms that are controlled via a remote console.38 By itself, this system has limitations in intraocular surgeries because the pivot point of the arms lies external to the eye. But, when combined with the Hexapod Surgical System (HSS), the Da Vinci system can overcome its remote center-of-motion problem.34,36,37
The HSS is able to place the remote center of motion at the site of ocular penetration, as opposed to a site external to the eye.37 The HSS consists of a main platform and six linear actuators that change length in accordance with the Da Vinci robot’s instructions.34,35
The combined use of OCT and robotics is inevitable in vitreoretinal surgeries.35 In the future, it should be possible to relay information gleaned from OCT images directly to the robotic surgical instrument.35
Postoperative Considerations
Postoperative management includes visual acuity and IOP evaluations as well as an assessment of the air/gas/silicone bubble on indirect biomicroscopy. Atropine 1% q.d. to b.i.d. and prednisolone acetate 1% b.i.d. to q.i.d. should be used to manage ocular inflammation and associated discomfort. Additional topical medication may be implemented to reduce elevated IOP or provide infection prophylaxis.
Because vitrectomy surgery almost always induces cataract development, patients must be informed that a second procedure (phacoemulsification with intraocular lens implantation) may be necessary to restore vision to optimal levels. Other complications of vitreomacular surgery include retinal breaks, rhegmatogenous retinal detachment, anterior hyaloidal fibrovascular proliferation, corneal decompensation, hypotony and endophthalmitis as well as elevated intraocular pressure secondary to neovascular glaucoma, angle closure, inflammatory debris, corticosteroid response or gas bubble overfill.38
VMT syndrome may exhibit mild to severe patient symptomology as well as a variable clinical presentation. Typically, a diagnosis is made upon fundoscopic examination with the assistance of medical imaging systems––particularly OCT.
Whenever VMT syndrome––or any of its variations––is observed, patients should be given an Amsler grid to monitor their status at home. Additionally, be sure to regularly schedule patients with VMT syndrome for dilated examinations to rule out the need for interventional procedures.
Ms. Joyce is a 4th year optometry student at the Pennsylvania College of Optometry at Salus University in Elkins Park, Pa. Dr. Gurwood is professor of clinical sciences at the Eye Institute at Salus University.
1. Smiddy WE, Maguire AM, Green WR, et al. Idiopathic epiretinal membranes. Ultrastructural characteristics in clinical pathologic correlation. Ophthalmology. 1989 Jun;96(6):811-20; discussion 821.
2. Shechtman DL, Dunbar MT. The expanding spectrum of vitreomacular traction. Optometry. 2009 Dec;80(12):681-7.
3. Takahashi A, Nagaoka T, Yoshida A. Stage 1-A macular hole: a prospective spectral-domain optical coherence tomography study. Retina. 2011 Jan;31(1):127-47.
4. Mann DF. Idiopathic macular hole. Optom Clin. 1996;5(1):95-110.
5. Gass JD. Vitreous traction maculopathy. In: Gass JD. Steroscopic Atlas of Macular Diseases. St Louis: Mosby; 1987:678-83.
6. Sharma RK, Ehinger BE. Development and structure of the retina. In: Kaufman PL, Alm A. Adler’s physiology of the eye: Clinical application. St Louis: Mosby; 2010:319-47.
7. Carr RE, Siegel IM. Fundamentals of electroretinography. In: Carr RE, Siegel IM. Electrodiagnostic testing of the visual system: a clinical guide. Philadelphia: F.A. Davis Company; 1990:3-8.
8. La Cour M. The retinal pigment epithelium. In: Kaufman PL, Alm A. Adler’s physiology of the eye: clinical applications. St. Louis: Mosby; 2010:319-47.
9. Lam BL. Full-field electroretinogram. In: Lam BL. Electrophysiology of vision: clinical testing and applications. Boca Raton, Fla.: Taylor & Francis; 2005:1-64.
10. Hutchinson JK, Gurwood AS. Clinical application of electrodiagnostic testing. Review of Optometry 7th annual guide to Retinal Disease. Rev Optom. 2010 Nov;147(11):suppl 4-6.
11. Hirokawa H, Jalk AE, Takahashi M, et al. Role of vitreous in independent preretinal macular fibrosis. Am J Ophthalmol 1989 Jan;101(1):166-9.
12. Green WR. Vitreoretinal juncture. In: Ryan SJ. Retina. St. Louis: Mosby; 1989:13-69.
13. Bainbridge J, Herbert E, Gregor Z. Macular holes: vitreoretinal relationships and surgical approaches. Eye (Lond). 2008 Oct;22(10):1301-9.
14. Kleinberg TT, Tzekov RT, Stein L, et al. Vitreous substitutes: A comprehensive review. Surv Ophthalmol. 2011 Jul-Aug;56(4):300-23. doi: 10.1016/j.survophthal.2010.09.001.
15. Skeie JM, Mahaian VB. Dissection of the human vitreous body elements for proteomic analysis. J Vis Exp. 2011 Jan 23;(47). pii: 2455. doi: 10.3791/2455.
16. Smiddy WE, Michels RG, Green WR. Morphology, pathology, and surgery for idiopathic macular disorders. Retina. 1990;10(4):288-96.
17. Gandorfer A, Rohleder M, Kampik A. Epiretinal pathology of vitreomacular traction syndrome. Br J Ophthalmol. 2002 Aug;86(8):902-9.
18. Smiddy WE, Michels RG, Glaser BM, deBustros S. Vitrectomy for macular traction caused by incomplete vitreous separation. Arch Ophthalmol. 1988 May;106(5):624-8.
19. Jaffe NS. Macular retinopathy after separation of vitreoretinal adherence. Arch Ophthalmol. 1967 Jan;78(1)585-91.
20. McDonald HR, Johnson RN, Schatz H. Surgical results in the vitreomacular traction syndrome. Ophthalmology. 1994 Aug;101(8):1397-402; discussion 1403.
21. Hikichi T, Yoshida A, Trempe CL. Course of vitreomacular traction syndrome. Am J Ophthalmol. 1995 Jan;119(1):55-61.
22. Koemer F, Garweg J. Vitrectomy for macular pucker and VMTS. Doc Ophthalmol. 1999;97(3-4):449-58.
23. Sulkes DJ, Ip MS, Baumal CR, Wu HK, Puliafito CA. Spontaneous resolution of vitreomacular traction syndrome documented by optical coherence tomography. Arch Ophthalmol. 2000 Feb;118(2):286-7.
24. Blana SA, Mubhoff F, Hoeller T, et al. Variations in vitreous humor chemical values as a result of pre-analytical treatment. Forensic Sci Int. 2011 Jul 15;210(1-3):263-70.
25. Chung EJ, Lew YJ, Lee H, Koh HJ. OCT-guided hyaloid release for vitreomacular traction syndrome. Korean J Ophthalmol. 2008 Sep;22(3):169-73.
26. Reese AB, Jones IR, Cooper WC. Vitreomacular traction syndrome confirmed histologically. Am J Ophthalmol. 1970 Jan;69(1):975-7.
27. Hashimoto E, Hirakata A, Hotta K, et al. Unusual macular retinal detachment associated with vitreomacular traction syndrome. Br J Ophthalmol. 1998 Jan;82(1):326-7.
28. Yamada N, Kishi S. Tomographic features and surgical outcomes of vitreomacular traction syndrome. Am J Ophthalmol. 2005 Jan;139(1):112-7.
29. Hassenstein A, Scholz F, Richard G. OCT in macular holes. Ophthalmologe. 2004 Aug;101(8):777-84.
30. Gregor ZJ. Surgery for idiopathic full-thickness macular holes. Eye (Lond). 1996;10 ( Pt 6):685-90.
31. Stalmans P, Delaey C, de Smet M, et al. Inravitreal injection of microplasmin for treatment of vitreomacular adhesion: Results of a prospective, randomized, sham-controlled phase II trial (The MIVI-IIT Trial). Retina. 2010 Jul-Aug;30(7):1122-7.
32. Benz MS, Packo KH, Gonzalez V, et al. A placebo-controlled trial of microplasmin intravitreous injection to facilitate posterior vitreous detachment before vitrectomy. Ophthalmology. 2010 Apr;117(4):791-7.
33. Freeman WR, Azen SP, Kim JW, et al. Vitrectomy for the treatment of full-thickness stage 3 or 4 macular holes. Results of a multicentered randomized clinical trial. Arch Ophthalmol. 1997 Jan;115(1):11-21.
34. Tsui I, Tsirbas A, Mango CW, et al. Robotic surgery in ophthalmology. In Tech. 2011:149-64.
35. Liu X, Balicki M, Taylor RH, Kang JU. Towards automatic calibration of Fourier-Domain OCT for robot-assisted vitreoretinal surgery. Opt Express. 2010 Nov 8;18(23):24331-43.
36. Bourla DH, Hubschman JP, Culjat M, et al. Feasibility study of intraocular robotic surgery with the da Vinci surgical system. Retina. 2008 Jan;28(1):154-8.
37. Mulgaonkar AP, Hubschman JP, Bourges JL, et al. A prototype surgical manipulator for robotic intraocular micro surgery. Stud Health Technol Inform. 2009;142:215-7.
38. Engelbert M, Chang S. Vitrectomy. In: Yanoff M, Duker J. 3rd ed. Philadelphia: Mosby; 2010:530-3.

