 A 31-year-old Hispanic male presented with longstanding blurred vision in his left eye that had persisted for four years. He did not know the cause of his reduced vision, but reported that it had remained stable. He demonstrated excellent vision in his right eye, however.
A 31-year-old Hispanic male presented with longstanding blurred vision in his left eye that had persisted for four years. He did not know the cause of his reduced vision, but reported that it had remained stable. He demonstrated excellent vision in his right eye, however.
His systemic history was significant for HIV. His CD4 count was in the mid-400s and he reported his viral load as “undetectable.” His current medications included Combivir (lamivudine/zidovudine, GlaxoSmithKline) and Sustiva (efavirenz, Bristol-Myers Squibb).
Best-corrected visual acuity measured 20/20 O.D. and 20/400 O.S. through eccentric viewing. Confrontation fields were full to careful finger counting O.U. His pupils were equally round and reactive, with trace afferent pupillary defect O.S. The anterior segment examination of both eyes was unremarkable. Intraocular pressure measured 12mm Hg O.U.
Dilated fundus exam of the right eye was completely normal. The left eye showed trace vitreous cells in the anterior vitreous. The optic nerve had a small cup, with good rim coloration and perfusion. The left macula demonstrated marked changes (figure 1). We also performed optical coherence tomography on his left eye (figure 2).
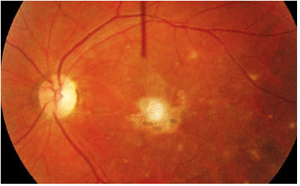
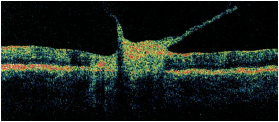
1,2. Posterior pole and macula of our patient’s left eye (left). What do you see? A vertical OCT cut through the left macula (above).
We closely followed the patient for several months, but subsequently lost him to follow-up. Then, he returned approximately four years later for a routine evaluation. His medical history remained unchanged, and he still took the same medications. Unfortunately, he did not know his CD4 count or viral load. His visual acuity remained 20/20 O.D. and 20/400 O.S. He still had trace vitreous cells in his left eye. The posterior pole and macula revealed significant changes (figure 3).
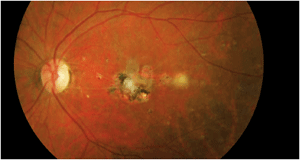
3. Our patient's left eye four years later. Note the changes in his posterior pole.
Take the Retina Quiz
1. What does the lesion in his macula represent?
a. Old chorioretinal scar.
b. Active retinochoroiditis.
c. Vitreomacular traction (VMT) syndrome.
d. Choroidal neovascularization (CNV).
2. What is the likely cause of the fundus lesion?
a. Toxocara canis.
b. Toxoplasmosis.
c. Cytomegalovirus (CMV) retinitis.
d. Herpes zoster ophthalmicus (HZO).
3. How should this patient be managed?
a. Observation.
b. Anti-vascular endothelial growth factor (VEGF) therapy.
c. Antiviral therapy.
d. Anti-toxoplasmosis medication.
4. How did the fundus lesion change between initial presentation and four-year follow-up?
a. It did not change.
b. The eye is now quiet.
c. There is evidence of continued activity.
d. There is active bleeding.
5. What is NOT an appropriate treatment for this fundus lesion?
a. Sulphonamides, such as sulfadiazine and sulfamethazine.
b. Ganciclovir.
c. Daraprim (pyrimethamine, GlaxoSmithKline).
d. Clindamycin.
For answers, see below.
Discussion
The macular lesion seen at initial examination four years ago represents a smoldering, active toxoplasmosis. Cells are present in the anterior vitreous and seen overlying the lesion. Additionally, the lesion has a white, “fluffy” appearance characteristic of an active toxoplasmosis retinochoroiditis. Its granular presentation signifies that the lesion may be resolving and forming a scar.
Of particular interest, the OCT revealed dense vitreous bands adherent to the macula that were causing traction. Also, his macula appears elevated, which likely represents an active infection as well as a swollen retina.
Toxoplasmosis is caused by an intracellular parasite, Toxoplasma gondii. Active toxoplasmosis retinochoroiditis typically presents as a feathery, white or creamy-yellow lesion. The lesion may appear thick or have a slightly elevated appearance. Other findings often include vitritis, papillitis, perivasculitis and sheathing of the retinal vessels.1
Anterior chamber cells may be common due to a “spill-over” effect from the active vitritis. The hazy media from the vitritis and creamy, white color of the active lesion results in the classic “headlights in the fog” appearance.
Interestingly, however, our patient does not exhibit the dense vitritis commonly associated with most cases of active toxoplasmosis. One explanation for this may be that because he is HIV-positive, he may not have a strong enough immune system to induce such an inflammatory reaction. However, the patient had a CD4 in the mid-400s. So, he should have been sufficiently healthy to develop notable inflammation. The other possible explanation may have been that the active toxoplasmosis infection was more indolent in nature, yielding a suppressed inflammatory response.
Clearly, this specific case involves a virulent organism, because four years elapsed with continued smoldering activity. The macular lesion developed more scar tissue as well as several smaller focal scarred lesions around the macula that were not present four years earlier. Further, there seems to be an active, yet resolving, “white” lesion located approximately one disc diameter temporal to the macula.
It was once believed that most active infections represented reactivation of congenital toxoplasmosis. Today, however, it is recognized that active toxoplasmosis infections are acquired later in life.1 Acquired cases of toxoplasmosis may be transmitted via unwashed vegetables, uncooked or undercooked meats, unpasteurized bovine milk, and even contaminated drinking water. Contact with contaminated soils and litter boxes may also be a source of acquired toxoplasmosis.1
In the congenital form of toxoplasmosis, patients generally present with an inactive chorioretinal scar. Typically, these scars are located in the posterior pole or directly in the macula, as was the case in our patient. Reactivation of a congenital infection usually occurs adjacent to an old scar. In fact, at our patient’s initial examination, we noted two smaller pigmented scars located inferior to the fluffy, white macular lesion that likely represent congenital infections or prior episodes of active disease.
Acquired toxoplasmosis infection often presents unilaterally as a solitary lesion and exhibits a predilection for the mid-peripheral fundus. Reactivation of an acquired infection can also occur. Like the congenital form, a reactivated acquired infection often occurs adjacent to the original infection. So, an acquired reactivation can appear similar or identical to a congenital reactivation.
It was difficult to determine if our patient has a reactivated congenital infection or reactivated acquired infection. Further, there was speculation on the relationship between his HIV and the toxoplasmosis. Unfortunately we were not able to draw any conclusions.
So, how should this patient be managed? Toxoplasmosis is a self-limiting disease in healthy patients; however, uveitis specialists agree that there are certain indications for treatment.1,2 These include an area of active retinitis threatening either the macula or the optic nerve, or a significant reduction in visual acuity due to a severe vitritis in an active lesion located away from the macula or optic nerve. Other indications for treatment include active lesions greater than one disc diameter in size and/or infection in immunocompromised patients.1,2 Generally, peripheral lesions that do not affect visual acuity can be followed without treatment.
Upon presentation, our patient’s macula was already directly involved, so there was not much hope of recovering central vision. Nonetheless, given the lesion’s central location and the patient’s medical history, we recommended treatment.
The classic treatment regimen for ocular toxoplasmosis consists of “triple drug therapy”––Daraprim, sulfadiazine and corticosteroids. Other treatments regimens include the use of clindamycin and/or azithromycin in conjunction with sulfadiazine and prednisone.1,2 Remember, corticosteroids must not be used alone due to a risk for recurrent infection.
Our patient was treated at initial examination, but became noncompliant and stopped his therapy. At four-year follow-up, we recommended further treatment. We will continue to follow this patient.
1. Jabs, DA, Nguyen QD. Ocular Toxoplasmosis. In: Ryan SJ, Schachat AP, Murphy RP (eds). Retina, vol. 2: Medical Retina, 4th ed. St. Louis: Mosby; 2006:1583-95.
2. Engstrom R, Holland G, Nussenblatt R, Jabs D. Current practices in the management of ocular toxoplasmosis. Am J Ophthalmol. 1991 May 15;111(5):601-10.
Retina Quiz Answers: 1) b; 2) b; 3) d; 4) c; 5) b.

