 |
History
A 67-year-old black female reported to the office with a chief complaint of vision changes OS of four weeks’ duration. She reported noticing a cloudiness after waking and that the reduction in clarity continued to worsen over the next three weeks. Her systemic history was remarkable for hypertension, diabetes and dyslipidemia which was well controlled with appropriate oral medications.
Her ocular history was remarkable open angle glaucoma controlled with prostaglandin monotherapy and an uncomplicated non-ischemic central vein occlusion OS, which had occurred a year earlier and resolved without requiring ocular intervention. She denied allergies of any kind.
Diagnostic Data
Her best-corrected entering visual acuities were 20/20 OD and 20/70 OS at distance and near with no improvement upon pinhole. Her external exam was normal with no evidence of afferent pupil defect. The biomicroscopic exam of the anterior segment was normal with no evidence of iris neovascularization. Goldmann applanation tonometry measured 15mm Hg OU. The pertinent findings are demonstrated in photographs. We also noted peripheral pathologies in both eyes.
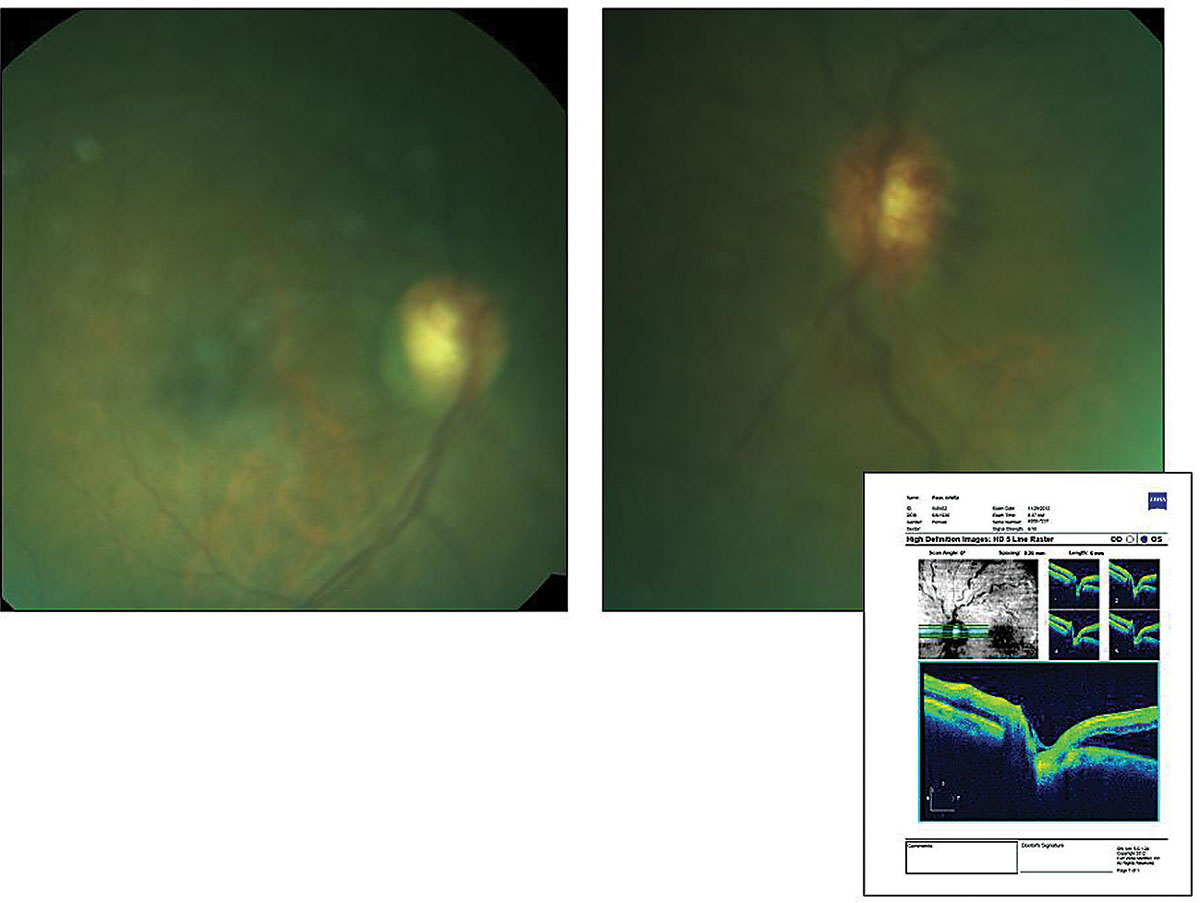 |
| This 67-year-old patient has a history of open-angle glaucoma and an uncomplicated non-ischemic central vein occlusion. She also had hypertension, diabetes and dyslipidemia. Does this presentation plus her history help identify the cause of her reduced visual clarity? Click image to enlarge. |
Discussion
Additional studies included indirect stereo-biomicroscopic examination of the fundus, color and brightness testing to rule out optic nerve involvement, photdocumentation and optical coherence tomography (OCT).
The diagnosis in this issue is non-ischemic sequential central venous occlusion OS with macular edema. Retinal vein occlusion (RVO) is a commonly encountered intraretinal vascular event.1-3 Reports on retinal vasculopathy recognize its prevalence second only to diabetic retinopathy.2,3 Patients who are symptomatic, typically present with a chief complaint of painless blurry vision in one eye, although vein occlusion can occur bilaterally.1-4 Since the disease itself is often the result of the systemic and local genetic factors that induced the event, there is no gender predilection, there is no racial preference and there are no concrete epidemiologic data as that information is subcategorized to the specific diseases that increase its risk.5
Retinal vein occlusions are categorized as those that permit vascular perfusion (nonischemic) and those that produce vascular stasis, limiting blood circulation (ischemic). Patients who present with large, ischemic branch retinal vein occlusions may present with an afferent papillary defect. Patients with ischemic retinal vein occlusions where the macula is involved may have significantly reduced visual acuity with a poorer prognosis then their nonischemic counterparts.6 In contradistinction, patients presenting with nonischemic branch retinal vein occlusions may have minimal to no functional interruptions. In general, retinal vein occlusions has a good prognosis.1-6
The retinal observations seen in retinal vein occlusion include dilated, tortuous retinal veins, sectoral, hemi-retinal or 4-quadrant flame-shaped intraretinal hemorrhages, variable local patches of retinal ischemia (cotton wool infarcts), intraretinal exudates, and variable retinal and or macular edema.3,6 The site of the occlusion can typically be observed at or just adjacent to the site of an arteriovenous crossing where retinal arteries and veins share a common adventitial sheath.1 Here, an increased (broadened) retinal arteriolar reflex can be seen connoting ongoing general vascular arteriolar and atheromatous vascular decompensation along with venous nicking, representing the mechanical forces applied by the overlying vessel.3,7 Complications which can be observed following a retinal vein occlusion include variable retinal edema, macular edema, capillary nonperfusion, retinal neovascularization, vitreous hemorrhage and tractional retinal detachment.7
Upwards of 50%-60% of eyes recover a final visual acuity of 20/40 or better without treatment.8 The entity rarely occurs idiopathically, rather it is frequently associated with systemic coagulopathy, hyperviscosity, infection, inflammation, hypertension, diabetes mellitus, dyslipidemia, antiphospholipid antibody syndromes, cardiac and carotid etiologies.1-9 Since most branch retinal vein occlusions resolve without sequellae, requiring no treatment, the most important initial step in formulating a management plan is to commit to discovering the underlying cause.1-9
The provoking mechanism of retinal vein occlusion is classically related to disorders of the blood tissue (blood dyscrasias) which incite platelet aggregation through coagulopathy syndromes and/or hyperviscosity syndromes or by mechanical mechanisms (venous compression secondary to an overlying artery’s expansion and hardening via atheromatous formation) which extinguishes forward venous blood flow.3,7 This causes the venous wall to be overcome by the forces of an abnormal volume with in its lumen. As the walls of the vessel decompensate, deoxygenated venous blood gains access to the retina via the nerve fiber layer, where the large caliber retinal veins reside.3,7 This produces an inflammatory and immunological reaction in the affected tissue.1,6 The discontinuation of venous blood flow also interrupts the arterial blood flow; blocked venous egress leaves no direct pathway for the venous blood or arterial blood to proceed.3-7 The overall flow in both systems becomes sluggish as the blood is forced through other anastamoses around the formed thrombus.1-11 The deoxygenated blood introduced into retinal tissues, along with other chemokines, cytokines and cellular players initiates tissue demolition in preparation for repair.1,5-9 These active substances as well as the setting of an ischemic environment can provoke the release of a vasoproliferative messengers known as vascular endothelial growth factors (VEGF).12,13 This category of substances stimulates new vessel growth by inducing neovascular “budding” off of existing arterial tissues. These new vessels lack normal architecture, become “scaffolded” to the vitreous body and have the potential to cause pre-retinal or vitreous hemorrhage and tractional retinal detachment.14
Since the pathophysiology of the event itself is painless, the symptomatology in these cases is directly related to interrupted function. In the initial stages, this is the direct effect of the light being blocked from reaching the photoreceptors. In cases where chronic, inner-retinal ischemia develops, capillary occlusion can result secondary to failed vascular “push.” Here increased hydrostatic (back) pressure in the vessels becomes transmitted to the capillary bed causing effected areas of the retina to become malnourished and hypoxic. This creates an environment conducive to retinal cellular death and persistent edema, both deleterious to function.7
Assessment
Retinal vein occlusion patients should be assessed both ocularly and systemically. While intraretinal neovascular formation is uncommon in cases involving branch retinal venous occlusion, ischemic processes are plausible, requiring the tissue to be observed through complete resolution.1 Systemic laboratory testing should include: blood pressure evaluation, complete blood count (CBC), Westergren sedimentation rate (E.S.R.), fasting treponemal antibody absorption test (FTA-Abs), reactive plasma regain (RPR), lipid profile and fasting blood sugar (FBS), homosystein, protein S and protein C, lupus anticoagulant, electocardiogram (EKG) and in appropriate cases carotid doppler testing.11 Infectious and inflammatory diseases have also been reported as sources for branch vein occlusion. Testing for sarcoidosis, tuberculosis, rheumatoid arthritris and lyme infection should be considered when vascular risks are small or laboratory work returns negative.15-17
While rare and dependent on the extent and location of the induced retinal ischemia, clinicians should monitor the IOP and iris for signs of rubeosis irides (iris/angle neovascularization).18 In the rare event a secondary glaucoma develops cycloplegics, topical steriods and oral/topical ocular hypotensives are required along with laser therapy.
The retinal treatment for all forms of vein occlusion is aimed at minimizing the loss of acuity from macular edema and preventing neovascularization.19-23 Clincal management for RVO is guided by the classic works of The National Institutes of Health, National Eye Institute. These include the Branch Retinal vein Occlusion Study (BRVOS), the Central Retinal Vein Occlusion Study (CRVOS N/M) and The Standard Care versus Corticosteriod Treatment for Branch Vein Occlusion (SCORE) studies and other studies known by their pneumonics as BRAVO, CRUISE, GALILEO and COPERICUS.21-23
Results from the 8-year BRVOS determined that prophylactic scatter argon laser photocoagulation (panretinal photocoagulation–PRP) would not prevent the development of neovascularization or vitreous hemorrhage in cases of BRVO and that macular argon laser photocoagulation (grid or focal photocoagulation) could improve visual acuity in eyes with persistent macular edema following branch retinal vein occlusion where vision was 20/40 or worse following the resolution of the event.21,22 Importantly, pan retinal laser photogogulation was found to be of benefit once neovasculaization was observed, significantly reducing the likelihood of vitreous hemorrhage by 30 %.21 This modality of treatment remains the standard of care.
The SCORE study was designed to compare the effectiveness and safety of between the standard care of BRVO (grid and focal laser photocoagulation) and intravitreal injection of triamcinolone for treating macular edema associated with both branch and central retinal vein occlusion (CRVO).23 The results of the trial found that there was no benefit for improving visual acuity for the steroid group compared to the standard care group in cases of BRVO.22 Interestingly, rates of adverse events (particularly elevated intraocular pressure and cataract) were increased in the steroid group receiving the 4mg injection.22 The study concluded that grid or focal photocoagulation should remain the standard of care for the treatment of macular edema secondary to BRVO.23
BRAVO, CRUISE, GALILEO and COPERNICUS have demonstrated that VEGF inhibitors can change the hydrodynamic forces within the retina helping to speed the resolution of macular edema as well as regress neovascularization. In a published report investigating 12-month follow-up results of intravitreal bevacizumab therapy for macular edema secondary to branch retinal vein occlusion24 a mean number of injections at 2, ranging from 1-4, the study demonstrated a benefit for all patients with younger patients having the best visual acuity improvement during and after the 12 month follow up period.24 Better pretreatment visual acuity was associated with better visual acuity at 12 months but with less improvement of the visual acuity.24 The researchers have opened the door for considering intravitreal bevacizumab alone or in combination with other accepted treatments as a long-term effective treatment for macular edema secondary to branch retinal vein occlusion.24
While elevated intraocular pressure (IOP), especially in patients with undiagnosed or poorly controlled glaucoma has been reported as a significant risk factor for developing central and hemiretinal venous occlusion, it seems to be less of a risk factor in the development or branch retinal vein occlusion.1,4 Lowering IOP provides little benefit in involved eyes whose IOP is already normal (10-22 mmHg).4 For completeness, variations of retinal vein occlusion include branch vain occlusion, vein occlusion of smaller venous tributaries (twig vein occlusion), an occlusion of one side of a bifurcated central retinal vein (hemiretinal vein occlusion) and occlusion of the entire central retinal vein (central vein occlusion).
Laboratory testing for inflammatory and infectious disorders might include an angiotensin converting enzyme study (ACE) and gallium scan for sarcoidosis, a chest X-ray (CXR) for tuberculosis and sarcoidosis, a lyme titre for lyme disease, antinuclear antibody testing for lupus and rheumatoid factor for arthritic disease.
| 1. Hayreh SS. Prevalent misconceptions about acute retinal vascular occlusive disorders. Prog Retin Eye Res. 2005;24(4):493-519. 2. Hamid S, Mirza SA, Shokh I. Branch retinal vein occlusion. J Ayub Med Coll Abbottabad. 2008;20(2):128-32. 3. Opremcak EM, Bruce RA. Surgical decompression of branch retinal vein occlusion via arteriovenous crossing sheathotomy: a prospective review of 15 cases. Retina. 1999;19(1):1-5. 4. Hayreh SS, Zimmerman MB, Beri M, et al. Intraocular pressure abnormalities associated with central and hemicentral retinal vein occlusion. Ophthalmology. 2004; 111(1):133-41. 5. Steinbrugger I, Haas A, Maier R, et al. Analysis of inflammation- and atherosclerosis-related gene polymorphisms in branch retinal vein occlusion. Mol Vis. 2009;15(3):609-18. 6. Margolis R, Singh RP, Kaiser PK. Branch retinal vein occlusion: clinical findings, natural history, and management. Compr Ophthalmol Update. 2006;7(6):265-76. 7. Funk M, Kriechbaum K, Prager F, et al. Intraocular concentrations of growth factors and cytokines in retinal vein occlusion and the effect of therapy with bevacizumab. Invest Ophthalmol Vis Sci. 2009;50(3):1025-32. 8. Bek T. Inner retinal ischaemia: current understanding and needs for further investigations. Acta Ophthalmol. 2009;87(4):362-7. 9. Rehak J, Rehak M. Branch retinal vein occlusion: pathogenesis, visual prognosis, and treatment modalities. Curr Eye Res. 2008;33(2):111-31. 10. Noma H, Funatsu H, Sakata K, et al. Macular microcirculation and macular edema in branch retinal vein occlusion. Br J Ophthalmol. 2009;93(5):630-3. 11. Mahoney BP, Constantine V. Retinal and Retinal Vascular Emergencies. In: Nyman, J. Problems in Optometry; Ocular Emergencies. JB Lippincott Co, Philadelphia, PA 1989: 123-48. 12. Chappell JC, Taylor SM, Ferrara N, et al. Local guidance of emerging vessel sprouts requires soluble Flt-1. Dev Cell. 2009;17(3):377-86. 13. Pournaras CJ, Rungger-Brändle E, Riva CE, et al. Regulation of retinal blood flow in health and disease. Prog Retin Eye Res. 2008;27(3):284-330. 14. Ho TY, Chung YM, Lee AF, et al. Severe vaso-occlusive retinopathy as the primary manifestation in a patient with systemic lupus erythematosus. J Chin Med Assoc. 2008;71(7):377-80. 15. DeRosa AJ, Margo CE, Orlick ME. Hemorrhagic retinopathy as the presenting manifestation of sarcoidosis. Retina. 1995;15(5):422-7. 16. O'Hearn TM, Fawzi A, Esmaili D, et al. Presumed ocular tuberculosis presenting as a branch retinal vein occlusion in the absence of retinal vasculitis or uveitis. Br J Ophthalmol. 2007;91(7):981-2. 17. Mikkilä HO, Seppälä IJ, Viljanen MK, et al. The expanding clinical spectrum of ocular lyme borreliosis. Ophthalmology. 2000;107(3):581-7. 18. Nahberger D, Meyer-Schwickerath R, Saygili O, et al. Development of neovascularization of the optic papilla, retina and iris. Dependence on site and extent of retinal ischemia. Ophthalmologe. 2000;97(6):422-8. 19. Heyreh SS. So-called "Central Retinal Vein Occlusion" II. venous stasis retinopathy. Ophthalmologica 1976; 172:14-35. 20. Heyreh SS, Heyreh MS. Hemi-Central Retinal Vein Occlusion. Pathogenesis, clinical features, and natural history. Arch Ophthalmol 1980; 98:1600-09. 21. Branch Vein Occlusion Study Group. Argon laser scatter photocoagulation for the prevention of neovascularization and vitreous hemorrhage in branch vein occlusion. Arch Ophthalmol 1986; 104:34-41. 22. Branch Vein Occlusion Study Group. Argon Laser Photocoagulation for Macular Edema in Branch Vein Occlusion. Am J Ophthalmol 1984; 98:271-282. 23. Scott IU, Ip MS, VanVeldhuisen PC, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with standard care to treat vision loss associated with macular Edema secondary to branch retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 6. Arch Ophthalmol. 2009;127(9):1115-28. 24. Kondo M, Kondo N, Ito Y, et al. Intravitreal injection of bevacizumab for macular edema secondary to branch retinal vein occlusion: Results After 12 Months and multiple regression analysis. Retina 2009; 29(7):913-925. |

