Annual Cornea ReportCheck out the other feature articles in this month's Cornea Report: Foreign Body Removal Start to Finish Understanding Corneal Nerve Function—and Dysfunction (Earn 2 CE) |
Patients with thin corneas susceptible to warpage and steepening had little more than corrective lenses and high-stake surgeries to turn to up until a few years ago. It’s no surprise, then, that corneal collagen crosslinking (CXL), approved in the United States in 2016, has been widely hailed as a quantum leap forward in the management of progressive keratoconus and, to a lesser extent, post-LASIK corneal ectasia.1
For the majority of patients, CXL slows disease progression and prevents further visual acuity loss, advanced disease and corneal transplantation, according to John Gelles, OD, of the Cornea and Laser Eye Institute (CLEI) and CLEI Center for Keratoconus in Teaneck, NJ. This subspecialty clinic is dedicated to researching and treating keratoconus and led the multicenter clinical trial for FDA approval of CXL in the United States.2,3 Although CXL is not intended to cure keratoconus, reduce its severity or improve vision, the latter two have been documented, albeit inconsistently, in the literature.1
“This treatment holds great therapeutic value for corneal diseases associated with reduced biomechanical strength, such as keratoconus or corneal ectasia following refractive surgery,” remarks Clark Chang, OD, of Wills Eye Hospital in Philadelphia. But doctors who practice in the United States are playing catch-up. “Due to the late adoption of CXL in the United States, clinical use of this important treatment is still slowly emerging,” says Dr. Chang.
As the procedure gains traction in the United States, researchers and clinicians are pushing forward with experimental new treatment protocols, techniques and indications. If you’re interested in comanaging these patients, here are insights from ODs and MDs well-versed in what CXL offers today and how it might evolve over time.
 |
UV light is applied to the eye of a CXL patient. Photo by Shawn Rocco, Duke Health. Click image to enlarge. |
Laying Down Roots
The corneal stromal can become thin either from pathologic processes (e.g., keratoconus, pellucid marginal degeneration) or iatrogenic ones, most notably LASIK surgery. Ectasia occurs when the thinning is severe enough to cause distension of the cornea—especially the cone-like bowing that gives keratoconus its name—resulting in refractive shift and, in severe cases, risks to the structural integrity of the eye. Crosslinking seeks to strengthen the bonds between the remaining collagen fibrils to mitigate this process. After bathing the cornea in riboflavin, a dose of UV light catalyzes a reaction that creates reactive oxygen species, which then form new covalent bonds among stromal fibrils. The cornea remains thin but, hopefully, emerges stronger than before.
First developed about 20 years ago in Europe, CXL has been a mainstay of treatment throughout Europe for the last 15 years and around the rest of the world for a decade, according to Christopher J. Rapuano, MD, chief of the Wills Eye cornea service. Drs. Rapuano and Chang have been involved with CXL research since 2008. At that time, the influx of data on success of the ‘epi-off’ technique, which requires debridement of the epithelium, in other countries led the United States to pursue this protocol for FDA approval. Epithelium removal improves penetration of riboflavin but, as can be expected, results in significant pain for patients during the healing phase.
In 2016, the KXL system from Avedro (now part of Glaukos) became the only FDA-approved collagen crosslinking device in the country thus far.4 The data that led to the device’s approval showed that, on average, the maximum keratometry values of patients with progressive keratoconus decreased by 1.60D one year after the procedure.2
Since then, the technique continues to find success in halting keratoconus progression in the majority of patients, with most studies suggesting control of progression in more than 90% of treated participants.5 A small percentage continue to experience progression after CXL, which Dr. Rapuano suggests could be due to eye-rubbing—a significant risk factor for progression—or severe disease.
Making Links
The procedure, while relatively simple, requires upwards of two hours of chair time for the patient, according to Dr. Rapuano. Here, he outlines the procedure steps he and his team follow:
- Numb the eye with topical anesthetic, instill antibiotic drops and lay the patient down.
- Sterilize the area around the eye with povidone-iodine, and apply an eyelid speculum.
- Use a semi-sharp blade to remove the epithelium (~10mm diameter, a technique known as epi-off or the Dresden protocol), and then remove the lid speculum.
- Administer riboflavin drops every two minutes for 30 minutes.
- Sit the patient up and, behind the slit lamp, check that the riboflavin has been absorbed throughout the cornea and into the anterior chamber.
- Lay the patient back down, and check corneal thickness. If the measurement is not at least 400µm, the next step is to apply hypotonic riboflavin drops every five to 10 seconds for as long as it takes the cornea to reach this parameter, which could mean an additional 20 minutes for some.
- Once the eye is prepped, apply ultraviolet (UV) light to the cornea for 30 minutes while administering riboflavin and numbing drops periodically. The care team must make sure the UV light is centered on the eye and the limbus is protected from any extraneous UV, advises Dr. Rapuano.
- Upon completion of the procedure, rinse off the riboflavin, put in a bandage contact lens and clean off the povidone-iodine.
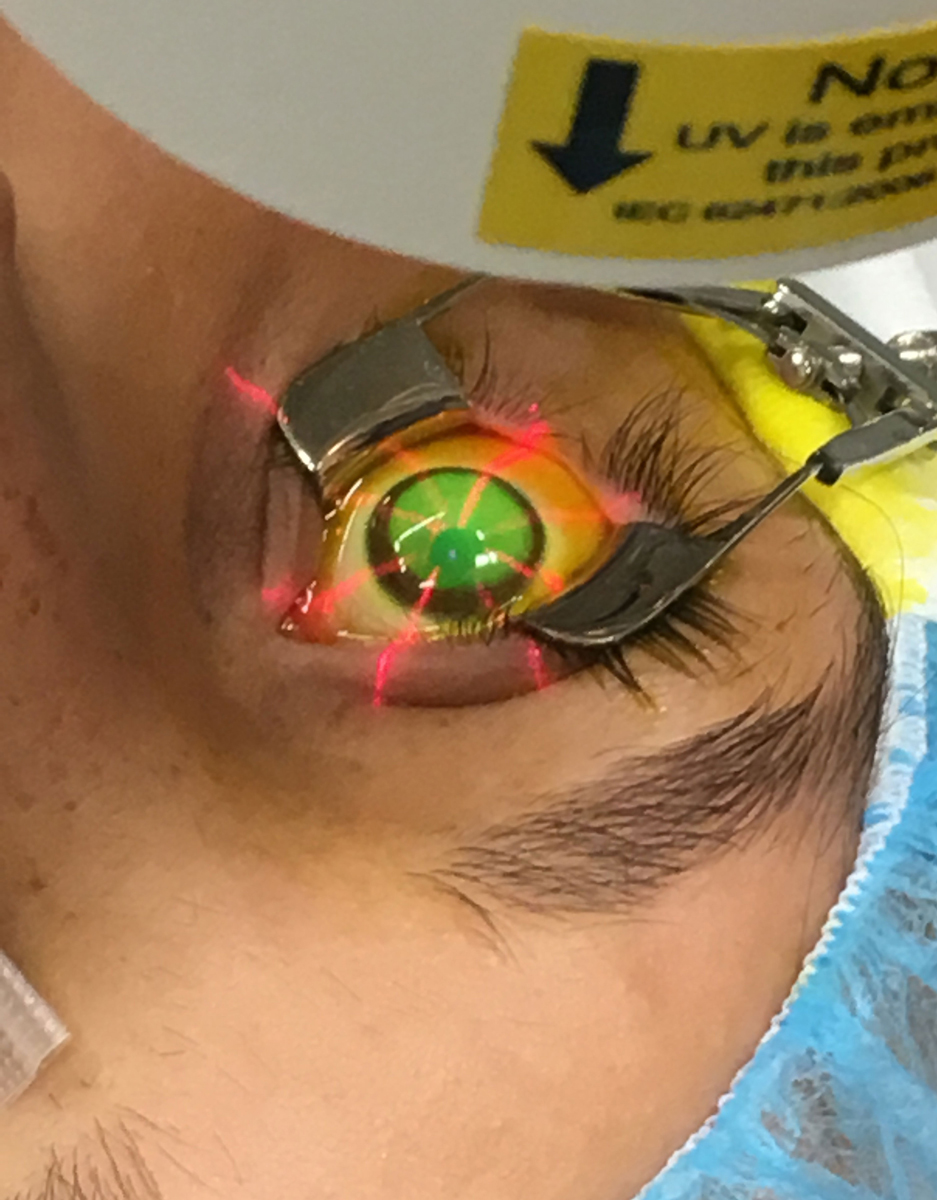 |
| In this part of the procedure, UV light is focused on the eye for 30 minutes. Photo by Glaukos. Click image to enlarge. |
Dr. Rapuano performs the entire procedure personally but may ask a technician to administer some of the riboflavin drops. He emphasizes the importance of operating the UV light device himself “because I think that’s kind of the critical part of the procedure.”
Patients should expect discomfort for a few days post-op, with vision returning to baseline within one to three months. During the first few postoperative days, Dr. Rapuano says it’s important that they use ice packs and over-the-counter pain medication, antibiotics and a bandage contact lens for the corneal epithelial defect and steroid drops for the inflammation.
Dr. Gelles, of CLEI—where some of the foundational US clinical trials on CXL were conducted by Drs. Peter Hersh and Steven Greenstein—suggests scheduling follow-ups at one day, four to five days and one, three, six and 12 months.6-14 He notes the first visit is aimed at ensuring there are no obvious complications, while the second is to remove the bandage lens and confirm epithelial healing. The later visits allow clinicians to evaluate changes to baseline measurements.
“Though rare, we have seen patients who have been lost to follow-up after CXL treatment only to return with a more advanced disease state,” says Dr. Gelles. “The need for appropriate follow-up on these patients cannot be overstressed. Continuous monitoring, despite treatment with CXL, is extremely important.”
Shortcomings and Complications
The current technique has seen some updates to shorten procedure duration, reduce recovery time and create a more robust effect. Still, it’s not perfect.
“Removal of the epithelial tight junction was thought to permit better riboflavin penetration into the corneal stroma, although it also increases the risks for potential complications,” according to Dr. Chang. While the benefits of CXL are well documented, the technique is associated with certain drawbacks that, when not managed appropriately, may mean the difference between a satisfied patient and an unsuccessful outcome.
Procedural risks could include infectious or non-infectious keratitis, corneal haze or opacity and persistent epithelial defects, says S. Barry Eiden, OD, a keratoconus specialist from Chicago and president of the International Keratoconus Academy. He adds that some patients may struggle with the post-op healing process and experience challenges with discomfort or pain, slow visual recovery, corneal shape changes requiring a contact lens or spectacle refitting and the initial inability to wear contact lenses.
The riboflavin solution causes stromal dehydration, which elevates the risk of scarring, swelling and melting; Dr. Rapuano includes these possibilities in what he characterizes as a 1% chance of complications. “That 1% is why we don’t do CXL on everyone with keratoconus who walks in,” he says. “If they’re not progressing or getting worse, then we don’t want to take even a small 1% risk of creating a bigger problem.”
CXL may halt disease progression, but it doesn’t answer the patient’s visual needs, Brian Chou, OD, of San Diego, notes. “CXL is a defensive play against progression, while specialty contact lens care is an offensive play to bring out maximal vision,” he remarks. This is why it is important for the patient and practitioner to carefully weigh the benefits and risks of CXL.
According to Dr. Chou, improving practitioner understanding and setting patient expectations go hand-in-hand for success with CXL. Some advocates overlook the fact that the procedure may not help keratoconus patients who have already achieved disease stability in older age or those with advanced distortion, he says. Those in their teenage years to early 20s whose keratoconus is detected soon after onset stand to reap the greatest value of CXL, explains Dr. Chou.
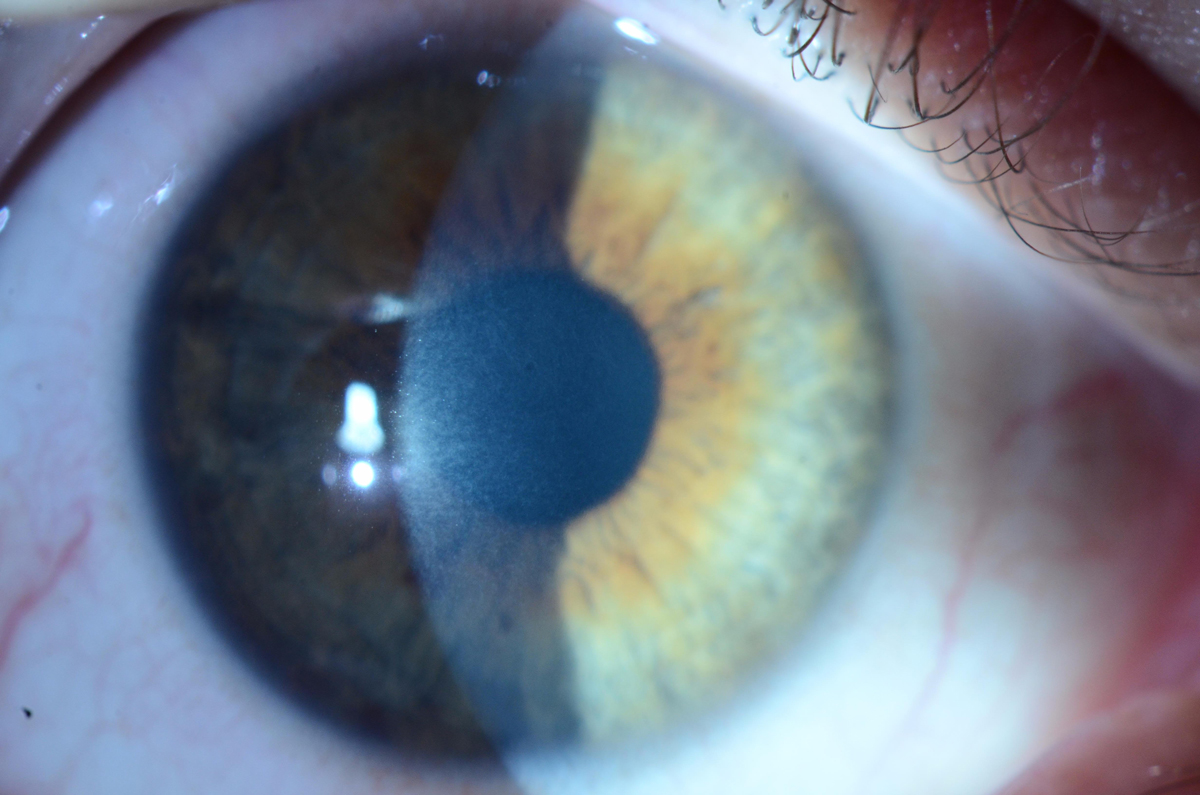 |
| Mild stromal haze can be seen one month after epi-off CXL.Photo by Christopher J. Rapuano, MD. Click image to enlarge. |
Potential Improvements
The rest of the world remains a few steps ahead of the United States, with access to protocols that allow transepithelial (or ‘epi-on’) CXL, accelerated CXL (which delivers higher doses of UV over a shorter time period) and other already well-known procedure options. But these are most likely headed to our shores eventually. “Now that epi-off CXL study data is well-established, many clinical trials are investigating the comparative treatment efficacy of different delivery protocols with the purpose of improving the overall patient experience,” Dr. Chang notes.
Here’s a quick look at each:
Epi-on. One study found that an investigational crosslinking protocol avoiding epithelium removal can safely stop disease progression in corneas as thin as 302nm and may offer faster visual recovery than other methods. The researchers used a new riboflavin formulation and application technique and a pulsed dosing of UVA to allow better oxygen transmission into the cornea.15
They observed improvements in uncorrected and corrected distance visual acuities (DVA), total higher-order aberrations and coma and maximum keratometry values. There was no progression or loss of effect, nor did the team note any complications. Participants were only uncomfortable for 24 hours post-op, with blurred vision lasting two to three days and contact lens wear resuming within a week.15
Another study published around the same time confirmed the efficacy of epi-on CXL, indicating that this method significantly improved maximum keratometry values and uncorrected DVA over one year.16 In a different study, the same researchers reported that while corneal haze increased slightly one month after epi-on CXL, it returned to baseline between three and 12 months and was substantially less than that previously reported post-standard CXL.17
Again looking into oxygen flow—this time for the epi-off technique—a team found that increasing oxygen concentration around the cornea during UVA radiation can improve the efficacy of conventional CXL.18
Accelerated. Other studies are looking into protocols employing a stronger UV light for less overall treatment time. Conventional CXL uses 3mW/cm for 30 minutes, while research into the accelerated technique documents anywhere from 9mW/cm for 10 minutes to 30mW/cm for four minutes.19 Investigators found that both accelerated protocols and conventional CXL had similar visual acuities, refractive outcomes and stabilization rates, although more parameters showed significant improvements 12 months after the standard protocol. While their results are promising, they also indicate the need for a better understanding of the effects of accelerated CXL.19
Around the same time, another team confirmed that accelerated CXL is a contender for keratoconus treatment, finding that the technique can safely halt progression within 24 months.20 After two years of follow-up, they observed an increase in the number of eyes with a corrected DVA of 0.3logMAR and a significant reduction in maximum and mean keratometry values.20 Other researchers demonstrated that the accelerated procedure is also associated with less corneal haze and a smaller risk of continuous flattening.21
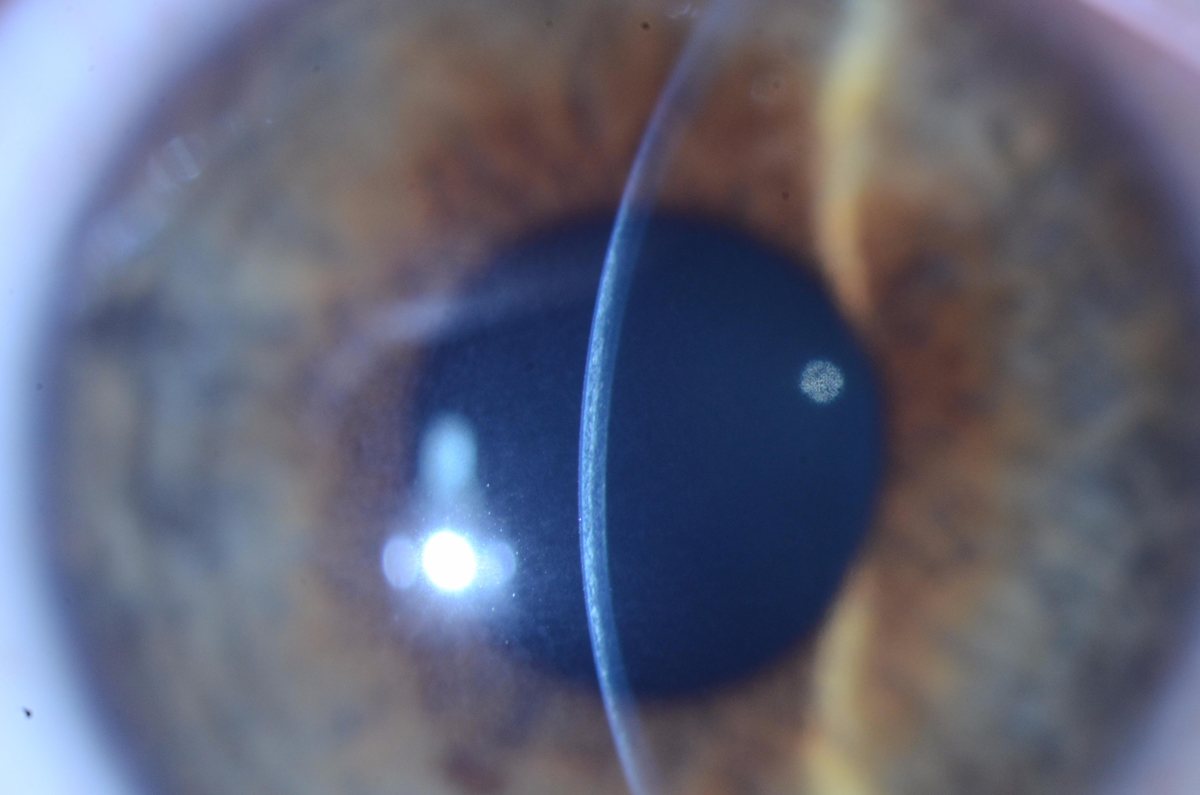 |
| The slit beam in this patient demonstrates a demarcation line at about 50% to 60% corneal depth. Photo by Christopher J. Rapuano, MD. Click image to enlarge. |
A new method known as custom fast CXL does not disrupt the epithelium and features 15 minutes of corneal presoaking with a riboflavin-vitamin E solution and a 370nm UVA radiation beam centered on the most highly curved region of the cornea. The study noted “a significant, rapid and lasting cone progression stoppage, astigmatism reduction and visual acuity improvement.”22
Accelerated CXL may also aid in keratitis therapy. Researchers have recommended a technique called photoactivated chromophore for keratitis (PACK) CXL to treat moderate-sized, therapy-resistant bacterial corneal ulcers.23 The same reactive oxygen species that create new covalent bonds within the stroma have the added benefit of killing pathogens in the treated area; also, the stronger cornea is more resilient against pathogenic attack.
Another group of investigators provided further support for adjuvant PACK-CXL, finding that the procedure can expedite the resolution of infectious keratitis with bacterial or fungal etiologies by reducing healing time and infiltrate size.24 PACK-CXL’s usefulness may extend to fungal keratitis treatment as well, especially when combined with voriconazole therapy, a different study proposed.25
Combinations. Accelerated CXL may not provide any added benefits when combined with LASIK, though. A team looking into the efficacy of combining the two did not find any difference between the combination procedure and conventional LASIK in uncorrected DVA or refractive stability.26
Other simultaneous procedures involve CXL and corneal rings or photorefractive keratectomy (PRK). These may present effective options for keratoconus and other corneal ectasias, researchers suggest. They found that the changes in corrected and uncorrected DVA and maximum keratometry values were significant for both combinations. The team concluded that combined CXL and ring implantation may be more effective for eyes with irregular astigmatism and worse corrected DVA, while CXL and PRK may be more useful for eyes with irregular astigmatism but better corrected DVA.27
A study that further evaluated the combination of CXL and intracorneal ring segments supported the notion that this procedure is safe and offers significant improvements in corneal topography. The researchers recommended thicker segment size and single segment placement for the best results.28
On the other hand, another study looking into CXL combined with topography-guided PRK warned against the swift adoption of this procedure for keratoconus after observing various complications and continuous progression post-op. Adverse events included corneal haze, primary herpes simplex keratitis, persistent epithelial defects and central corneal stromal opacity. Related to removal of the epithelium were post-op pain, photophobia, lacrimation, foreign body sensation and healing difficulties.29
Many Hands Make (UV) Light WorkSo far, only ophthalmologists can perform CXL and, as would be expected, most practitioners are cornea and refractive surgery specialists. Where the procedure stands within optometric scope of practice is a little hazier and depends on specific state licensure. Dr. Chou says there is speculation that if CXL expands to include an epi-on approach, it may fall within the scope of optometric care for some states. Eliminating the need to debride the cornea raises the potential for optometrists in all 50 states to qualify to perform CXL if further legistion is enacted. Even if optometrists are not performing the procedure themselves, Dr. Eiden believes they are crucial members of the treatment team. Early disease and progression detection, timely treatment referral and proper follow-up scheduling, post-op care and visual rehabilitation with contact lenses all fall within the scope of optometry and play a critical role in optimizing visual outcomes. |
Combination procedures may be beneficial beyond keratoconus, with investigators reporting that performing transepithelial phototherapeutic keratectomy simultaneously with accelerated CXL may be an effective treatment option for pellucid marginal degeneration. Among the study’s highlights were improvements in cylindrical value, spherical equivalent value, keratometry value, corneal thickness and the occurrence of myriad adverse events.30
A Bright Future Ahead
“I see the field of CXL moving in the direction of less invasive forms of treatment with better safety profiles, lower complication risks, faster visual recovery times and the ability to return to contact lens wear relatively quickly,” Dr. Eiden says. He suggests CXL refinement will improve the treatment’s predictability and make it a good option for combination procedures.
In addition to anticipating an epi-on technique for United States CXL procedures, which would significantly improve post-op comfort, Dr. Chou foresees other advances, such as use of iontophoresis to draw riboflavin into the corneal stroma and variable patterns of UV light with intraoperative oxygen supplementation to enhance crosslinking. Avedro currently markets this overseas as photorefractive intrastromal crosslinking (PiXL).
Dr. Chang adds that CXL could eventually incorporate topography-guided technology to deliver different treatment plans across different corneal areas, which may produce more corneal regularization and refractive benefits. Avedro calls this the customized remodeled vision (CuRV) in international markets.
As for the prospect of epi-on CXL with an accelerated and pulsed UV light device, Dr. Chang believes that by keeping most of the epithelium intact and shortening CXL treatment time with a higher-energy light, patients may be more comfortable during the early post-op period and have a quicker visual recovery. Dr. Rapuano, however, mentions significant issues with epi-on that must be overcome first, including how the epithelium prevents riboflavin absorption, UV light transference and oxygen transmission, which are all necessary for CXL success.
CXL has come a long way from its humble origins in 1997.31 New protocols, techniques and indications are shifting the procedure out of the periphery and into the mainstream. They may, one day, also move the procedure within an expanded scope of optometric practice. Now’s the time to start preparing for this possibility.
1. Belin MW, Lim L, Rajpal RK, et al. Corneal cross-linking: current USA status: report from the Cornea Society. Cornea. 2018;37(10):1218-25. 2. Hersh PS, Stulting RD, Muller D, et al. United States multicenter clinical trial of corneal collagen crosslinking for keratoconus treatment. Ophthalmology. 2017;124(9):1259-70. 3. Hersh PS, Stulting RD, Muller D, et al. U.S. multicenter clinical trial of corneal collagen crosslinking for treatment of corneal ectasia after refractive surgery. Ophthalmology. 2017;124(10):1475-84. 4. Avedro. KXL system and Photrexa formulations. avedro.com/medical-professionals/corneal-cross-linking/kxl-system-and-photrexa-formulations/. Accessed March 11, 2020. 5. Hersh PS. The Cornea and Laser Eye Institute. U.S. multicenter clinical trial of corneal collagen crosslinking for treatment of corneal ectasia after refractive surgery. www.vision-institute.com/keratoconus-new-jersey/corneal-cross-linking/. Accessed March 11, 2020. 6. Greenstein SA, Fry KL, Bhatt J, et al. Natural history of corneal haze after collagen crosslinking for keratoconus and corneal ectasia: Scheimpflug and biomicroscopic analysis. J Cataract Refract Surg. 2010;36(12):2105-14. 7. Hersh PS, Greenstein SA, Fry KL. Corneal collagen crosslinking for keratoconus and corneal ectasia: one year results. J Cataract Refract Surg. 2011;37(1):149-60. 8. Greenstein SA, Shah VP, Fry KL, et al. Corneal thickness changes after corneal collagen crosslinking for keratoconus and corneal ectasia: one-year results. J Cataract Refract Surg. 2011;37(4):691-700. 9. Greenstein SA, Fry KL, Hersh PS. Corneal topography indices after corneal collagen crosslinking for keratoconus and corneal ectasia: one-year results. J Cataract Refract Surg. 2011;37(7):1282-90. 10. Greenstein SA, Fry KL, Hersh PS. In vivo biomechanical changes after corneal collagen cross-linking for keratoconus and corneal ectasia: 1-year analysis of a randomized, controlled, clinical trial. Cornea. 2012;31(1):21-5. 11. Greenstein SA, Fry KL, Hersh MJ, et al. Higher-order aberrations after corneal collagen crosslinking for keratoconus and corneal ectasia. J Cataract Refract Surg. 2012;38(2):292-302. 12. Brooks NO, Greenstein SA, Fry KL, et al. Patient subjective visual function after corneal collagen crosslinking for keratoconus and corneal ectasia. J Cataract Refract Surg. 2012;38(4):615-9. 13. Greenstein SA, Fry KL, Hersh PS. Effect of topographic cone location on outcomes of corneal collagen cross-linking for keratoconus and corneal ectasia. J Refract Surg. 2012;28(6):397-405. 14. Greenstein SA, Hersh PS. Characteristics influencing outcomes of corneal collagen crosslinking for keratoconus and ectasia: implications for patient selection. J Cataract Refract Surg. 2013;39(8):1133-40. 15. Stulting RD, Trattler WB, Woolfson JM, et al. Corneal crosslinking without epithelial removal. J Cataract Refract Surg. 2018;44(11):1363-70. 16. Hersh PS, Lai MJ, Gelles JD, et al. Transepithelial corneal crosslinking for keratoconus. J Cataract Refract Surg. 2018;44(3):313-22. 17. Lai MJ, Greenstein SA, Gelles JD, et al. Corneal haze after transepithelial collagen cross-linking for keratoconus: a Scheimpflug densitometry analysis. Cornea. April 8, 2020. [Epub ahead of print]. 18. Wang J, Wang L, Li Z, et al. Corneal biomechanical evaluation after conventional corneal crosslinking with oxygen enrichment. Eye Contact Lens. August 13, 2019. [Epub ahead of print]. 19. Lang PZ, Hefezi NL, Khandelwal SS, et al. Comparative functional outcomes after corneal crosslinking using standard, accelerated and accelerated with higher total fluence protocols. Cornea. 2019;38(4):433-41. 20. Ting DSJ, Rana-Rahman R, Chen Y, et al. Effectiveness and safety of accelerated (9mW/cm2) corneal collagen cross-linking for progressive keratoconus: a 24-month follow-up. Eye. 2019;33(5):812-8. 21. Kato N, Negishi K, Sakai C, et al. Five year outcomes of corneal collagen crosslinking: accelerated crosslinking induces less corneal haze and less continuous corneal flattening compared to conventional crosslinking. ARVO 2019. Abstract 313-B0505. 22. Caruso C, Epstein RL, Troiano P, et al. Topography and pachymetry guided, rapid epi-on corneal crosslinking for keratoconus: 7-year study results. Cornea. 2020;39(1):56-62. 23. Knyazer B, Krakauer Y, Baumfeld Y, et al. Accelerated corneal cross-linking with photoactivated chromophore for moderate therapy-resistant infectious keratitis. Cornea. 2018;37(4):528-31. 24. Ting DSJ, Henein C, Said DG, et al. Photoactivated chromophore for infectious keratitis-corneal cross-linking (PACK-CXL): a systematic review and meta-analysis. Ocul Surf. 2019;17(4):624-34. 25. Özdemir H, Kalkancı A, Bilgihan K, et al. Comparison of corneal collagen cross-linking (PACK-CXL) and voriconazole treatments in experimental fungal keratitis. Acta Ophthalmol. 2019;97(1):e91-6. 26. Kohnen T, Lwowski C, Hemkeppler E, et al. Comparison of femto-LASIK with combined accelerated crosslinking to femto-LASIK in high myopia eyes: a prospective randomized trial. Am J Ophthalmol. 2020;211:42-55. 27. Singal N, Ong Tone S, Stein R, et al. Comparison of accelerated crosslinking alone, accelerated crosslinking with simultaneous intrastromal corneal ring segments, and accelerated crosslinking with simultaneous topography-guided photorefractive keratectomy in progressive keratoconus and other corneal ectasias: a prospective non-randomized interventional study. J Cataract Refract Surg. 2020;46(2):276-86. 28. Hersh PS, Issa R, Greenstein SA. Corneal crosslinking and intracorneal ring segments for keratoconus: a randomized study of concurrent versus sequential surgery. J Cataract Refract Surg. 2019;45(6):830-9. 29. Iqbal M, Elmassry A, Tawfik A. Evaluation of the effectiveness of cross-linking combined with photorefractive keratectomy for treatment of keratoconus. Cornea. 2018;37(9):1143-50. 30. Cagil N, Sarac O, Yesilirmak N, et al. Transepithelial phototherapeutic keratectomy followed by corneal collagen crosslinking for the treatment of pellucid marginal degeneration: long-term results. Cornea. 2019;38(8):980-5. 31. Spörl E, Huhle M, Kasper M, et al. Increased rigidity of the cornea caused by intrastromal cross-linking. Ophthalmologe. 1997;94(12):902-6. |

