 |
Polycystic ovary syndrome (PCOS) is a complex condition characterized by menstrual irregularities, androgen excess and ovarian cysts.1 It is defined as the presence of any two of the following: polycystic ovaries, anovulation or oligoovulation and hyperandrogenism.2
A Triad of Signs
A woman has polycystic ovaries if 20 or more follicles—functional units of ovaries that secrete hormones—are present in at least one ovary. Ultrasonography can detect polycystic ovaries, which typically involve 10 to 12 small follicles spaced close together in a bilateral formation on the peripheral edge of the ovaries.2,3
Anovulation occurs when the ovaries do not release an oocyte during the menstrual cycle, precluding ovulation; however, a woman may still menstruate. If ovulation is present but irregular, it’s called oligoovulation (fewer than nine menstrual cycles per year).1,4
Hyperandrogenism—defined by hirsutism, excess of blood testosterone (T) levels or both—is an endocrine disorder that affects 5% to 10% of women of reproductive age.5 Clinical manifestations include hirsutism, acne, androgenic alopecia and virilization.1,4 Many of these patients also tend to have PCOS.
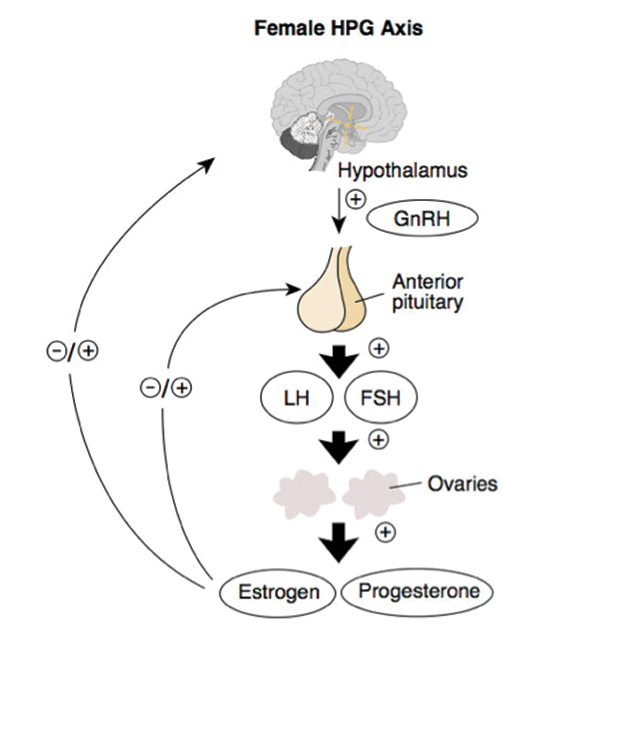 |
| Fig. 1. The HPG axis runs a feedback loop that could cause PCOS if interrupted. Click image to enlarge. |
PCOS Essentials
At least 7% of all adult women have PCOS, with one study estimating this number to be as high as 21%.6,7 It impacts approximately five million women of childbearing age in the US alone.3 Researchers believe PCOS is caused by a disordered feedback loop between the ovaries and the hypothalamic-pituitary-gonadal (HPG) axis (Figure 1). This leads to deficits in the release of luteinizing hormone in the presence of follicle-stimulating hormone.1,2
The inappropriate gonadotropin secretion seen in PCOS is most likely a result of, rather than a cause of, ovarian dysfunction. Another consistent biochemical feature of PCOS is a raised plasma T level.8 This causes concomitant insulin resistance and compensatory hyperinsulinemia, increasing glucose intolerance. These patients tend to be overweight; nearly half of all women with PCOS are clinically obese.9 Many have obstructive sleep apnea (OSA), which adds to the risk of cardiovascular disease.10 Clinicians should ask OSA patients about excessive daytime somnolence and assess their cardiovascular risk by evaluating their body-mass index (BMI), fasting lipid and lipoprotein levels and metabolic syndrome risk.2,3,11
Women with PCOS have a higher rate of infertility and are at increased risk of pregnancy loss and complications, including gestational diabetes.1,3 They also have an increased prevalence of coronary artery calcification and thickened carotid intima media, which may be responsible for subclinical atherosclerosis.1,3 Approximately 10% of women with PCOS have type 2 diabetes, and 30% to 40% have impaired glucose tolerance by the age of 40.11 Perform glucose tolerance and glycosylated hemoglobin testing, regardless of a patient’s weight.
Ocular Comorbidities
Estrogen, progesterone and androgen receptors have been found in the cornea, lens, iris, ciliary body, retina, lacrimal glands, meibomian glands and conjunctiva. It is thus incumbent on ODs to identify potential signs of PCOS and its ocular comorbidities.12 These may include ocular dryness and/or itching, diabetic retinopathy, floppy lid syndrome, central serous chorioretinopathy, keratoconus and anterior ischemic optic neuropathy. Idiopathic intracranial hypertension (IIH), or pseudotumor cerebri, may be another.
IIH initially presents as bilateral optic disc edema of unknown cause (Figure 2). It predominantly affects women of childbearing age who are obese. The condition causes chronically elevated intracranial pressure and papilledema, which may lead to progressive optic atrophy and reduced visual function.13
Given that obesity is commonly associated with IIH and PCOS, there may be an association between the two. A study of 38 women with IIH found 15 also had PCOS and 24 were obese.13 The researchers proposed PCOS-driven obesity facilitates IIH after learning that increased intra-abdominal and cardiac filling pressures associated with obesity may result in a retrograde increase in cerebrospinal fluid pressure.13 Another study reported that out of 58 women with IIH, nine had PCOS, concluding that the prevalence of PCOS is higher in IIH patients.14
Beyond obesity, PCOS may be associated with IIH through an increased risk of thrombosis. High estrogen levels, common in PCOS and severe obesity, are thrombophilic and may play a role in the development of IIH, particularly in patients with coagulation disorders.13,14
 |
| Fig. 2. Bilateral optic disc edema is visible in a patient with IIH, OD on top. Photo by Leonard V. Messner, OD. Click image to enlarge. |
Diagnosis
When PCOS is suspected, first exclude all other disorders that could result in menstrual irregularity and hyperandrogenism, including adrenal or ovarian tumors, thyroid dysfunction, congenital adrenal hyperplasia, hyperprolactinemia, acromegaly and Cushing syndrome.2,3
Baseline screening includes:3,4
- Thyroid function tests
- Serum prolactin level
- Total and free T levels
- Free androgen index
- 17-hydroxyprogesterone level
- Urinary free cortisol and creatinine levels
- Low-dose dexamethasone suppression test
- Insulin-like growth factor 1 level
- Human chorionic gonadotropin level
Ovarian biopsy may histologically confirm PCOS; however, ultrasonography generally supersedes histopathologic analysis. An endometrial biopsy can evaluate for endometrial disease, such as malignancy.2,3
Treatment
Lifestyle changes combined with metformin can help with weight loss, which is recommended to reduce cardiovascular risks and complications associated with diabetes.2,3
Pharmacologic treatments are reserved for metabolic derangements, such as anovulation, hirsutism and menstrual irregularities. First-line medical therapy usually consists of an oral contraceptive to regulate menstruation and decrease the risk of endometrial cancer, which is associated with obesity and chronic anovulation (but not necessarily PCOS).2,3
If symptoms are not sufficiently alleviated, an androgen-blocking agent may be added. First-line treatments for ovulation induction when fertility is desired are letrozole and clomiphene citrate.2,3 Clomiphene and metformin can induce ovulation in patients seeking fertility but should not be used together.15 Metformin use can be continued into pregnancy and may decrease miscarriage rates.
Medications could include:1-3
- Hypoglycemic agents
- Selective estrogen receptor modulators
- Topical hair-removal agents
- Oral contraceptive agents
- Anti-androgens
- Topical acne agents
Surgical management of PCOS aims to restore ovulation. There are two types: laparoscopic ovarian drilling and ovarian wedge resection. Laparoscopic drilling uses electrocautery or a laser to destroy parts of the ovary and trigger ovulation. Wedge resection removes a section of ovary to help regulate menstruation and promote normal ovulation.1-3
PCOS is a common, lifelong health condition in women. These patients can develop serious health problems, especially if they are overweight, and must be managed accordingly. Even ODs have a part to play, as ocular comorbidities could be present.
1. Carmina E, Lobo RA. Polycystic ovary syndrome (PCOS): arguably the most common endocrinopathy is associated with significant morbidity in women. J Clin Endocrinol Metab. 1999;84(6):1897-9. 2. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19-25. 3. Ndefo UA, Eaton A, Green MR. Polycystic ovary syndrome: a review of treatment options with a focus on pharmacological approaches. P T. 2013;38(6):336-55. 4. Vause TDR, Cheung AP, Sierra S, et al. Ovulation induction in polycystic ovary syndrome. J Obstet Gynaecol Can. 2010;32(5):495-502. 5. Yildiz BO. Diagnosis of hyperandrogenism: clinical criteria. Best Pract Res Clin Endocrinol Metab. 2006;20(2):167-76. 6. Copp T, Jansen J, Doust J, et al. Are expanding disease definitions unnecessarily labelling women with polycystic ovary syndrome? BMJ. 2017;358:j3694. 7. Aubuchon M, Legro RS. Polycystic ovary syndrome: current infertility management. Clin Obstet Gynecol. 2011;54(4):675-84. 8. Barber TM, Franks S. Genetics of polycystic ovary syndrome. Front Horm Res. 2013;40:28-39. 9. Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016;37(5):467-520. 10. Tasali E, Van Cauter E, Ehrmann DA. Polycystic ovary syndrome and obstructive sleep apnea. Sleep Med Clin. 2008;3(1):37-46. 11. Ehrmann DA, Barnes RB, Rosenfield RL, et al. Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care. 1999;22(1):141-6. 12. Adıyeke SK, Karaca I, Yıldırım S, et al. Anterior segment findings in women with polycystic ovary syndrome. Turk J Ophthalm. 2017;47(1):24-7. 13. Glueck CJ, Iyengar S, Goldenberg N, et al. Idiopathic intracranial hypertension: associations with coagulation disorders and polycystic-ovary syndrome. J Lab Clin Med. 2003;142(1):35-45. 14. Avisar I, Gaton DD, Dania H, et al. The prevalence of polycystic ovary syndrome in women with idiopathic intracranial hypertension. Scientifica. July 30, 2012. [Epub ahead of print]. 15. Wellberry C. Diagnosis and treatment of polycystic ovary syndrome. Am Fam Physician. 2007;76(6):865-76. |

