 |
A 70-year-old African-American female presented to the office with complaints of progressively reduced vision OS>OD of six months’ duration. She had an ocular history of proliferative diabetic retinopathy (PDR) with macular edema OU. She had been treated with panretinal photocoagulation (PRP) OU five years prior. She also underwent three intravitreal injections of bevacizumab (Avastin, Genentech) over the course of five years.
The patient explained that she had developed a vitreous hemorrhage and tractional retinal detachment in her right eye and underwent pars plana vitrectomy with retinal detachment repair using a silicone oil tamponade one year ago. The silicone oil was removed after six months and an oil-gas exchange was performed with sulfur-hexafluoride-6 (SF6) gas. Her vision has progressively worsened since that operation.
Her systemic history was remarkable for controlled hypertension and type 2 diabetes. She had no medical or environmental allergies. Both her family and social history were noncontributory.
The patient’s best-corrected entering visual acuity was hand motion OD and 20/40 OS through a mild myopic spectacle prescription. Her vision did not improve with pinhole testing OU. Her external testing was unremarkable and there was no afferent pupillary defect. Refraction did not improve acuity.
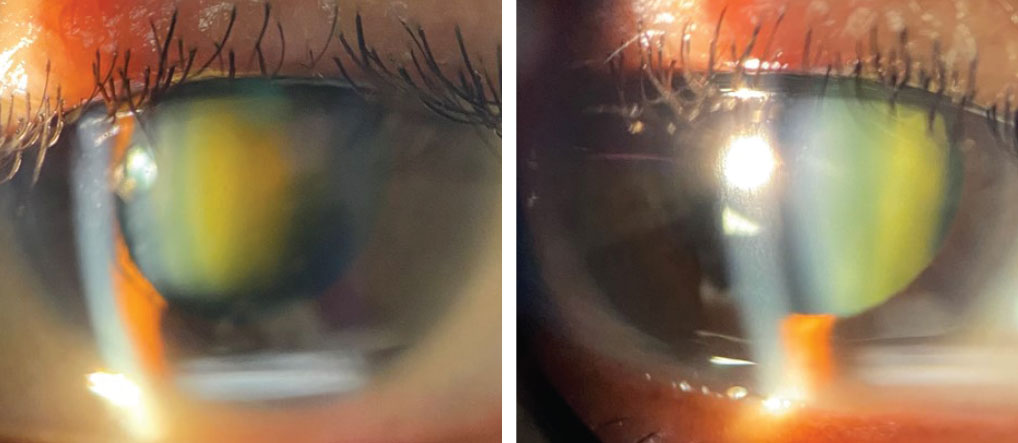
Slit lamp biomicroscopy was remarkable for a grade 3 nuclear cataract OD and a grade 2 nuclear cataract OS. There was no iris neovascularization. Her intraocular pressures (IOP) measured 14mm Hg OD and 15mm Hg OS with Goldmann applanation.
 |
|
Slit lamp biomicroscopy findings of the patient’s right and left eyes. Why might this presentation appear in a patient with the medical history described here? Click image to enlarge. |
Dilated fundus examination revealed very hazy views OD with old vitreoproliferative scarring OU. The OS demonstrated clear media with scattered moderate nonproliferative diabetic retinopathy (NPDR) and no new evidence of PDR. Her optic nerves were flat and perfused, with a cup-to-disc ratio of 0.4/0.4 OU. PRP scars were seen 360° OU. She was referred for cataract extraction, OD>OS.
Additional Testing
Laser interferometry was done to understand the patient’s macular potential. OCT of the macula was also completed to rule out the need for intervention regarding diabetic macular edema. Color fundus photographs were taken to record the status of both posterior poles.
Discussion
The diagnosis in this month’s issue is post-vitrectomy cataract development. Cataract progression is a common complication of pars plana vitrectomy (PPV) that can prolong visual recovery.1
Cataract extraction is often required within two years of PPV, as the vitreous’s absence induces premature lens opacification that not only reduces the patient’s vision but limits views of the retina, interrupting proper treatment of the posterior segment.2-4
From the patient’s perspective, the development of cataracts after the completion of a vitrectomy is a visual setback that delays their final visual rehabilitation. To circumvent this, a combined procedure can be performed where the lens is removed in the same procedure as the vitrectomy.3,5 This procedure is commonly referred to as a phacovitrectomy and it is frequently performed in vitrectomy cases where the cataract prevents adequate views of the retinal pathology. It may also be performed as an elective option in patients who present with the most risk of developing cataracts post vitrectomy procedure.5
The vitreous gel is a large bag of collagen fibrils that originate anteriorly at the vitreous base and extend posteriorly to fill most of the posterior chamber. The entire structure is encased in the hyaloid membrane which adheres to the retina at various points, principally the optic nerve and macula posteriorly and the vitreous base anteriorly.6
Surgical Procedure and Decision-Making
Vitrectomy is useful for a wide variety of vitreoretinal pathologies.7 It can be appropriate to remove vitreous when it becomes opaque from the induction of blood, inflammation or infection; to remedy tractional forces placed on the retina; as a prerequisite step for any procedure that requires mechanical manipulation of the retinal tissue such as retinal reattachment, epiretinal membrane removal, macular hole repair; or to address complications of anterior segment surgery.7
To perform the procedure, three “trochar cannulas” are inserted through the sclera. These guides permit the use of three instruments: a balanced saline solution (BSS) infusion line, a vitrector (vitreous cutting and vacuum instrument) and a fiber-optic probe (lighting source) to be introduced into the eye at the same time. The vitrector is then used to cut and aspirate the vitreous from inside the globe from the optic nerve to the vitreous base, depending on the needs of the case. When the entire vitreous is not removed, the procedure is known as a partial vitrectomy. A contact fundus lens is used to view the posterior vitreous during the procedure.
Any additional retinal procedures are performed afterward, such as neovascular membrane removal, epiretinal membrane removal or laser photocoagulation.7 The vitreous cavity may then be filled with BSS or, if a retinal tamponade is required, gas or silicone oil.7
In the combined procedure, the cataract extraction is performed first with a standard clear corneal phacoemulsification technique and insertion of a posterior chamber intraocular lens.
The incidence of post-vitrectomy cataract formation in the literature varies widely, from 20% 80%.1 There are numerous possible causes of post-vitrectomy cataract formation. Subcapsular lens opacities may temporarily form on the lens either intraoperatively or shortly after the vitrectomy. However, these usually resolve completely.1 A cataract may also form if the lens is touched with the vitrectomy instruments.6 The opacity develops rapidly and usually requires intraoperative removal.8
The vitreous acts as a barrier for diffusion of oxygen between the lens and the metabolically active retina. Removal of this barrier may accelerate oxidization of the lens proteins and produce subsequent cataract progression.9 This is supported by the fact that other vitreoretinal procedures that leave the vitreous intact such as pneumatic retinopexy have much lower rates of cataract formation.1,10 Another possibility is that the lack of contact of the posterior lens capsule with the vitreous impairs the metabolic exchange of the lens that is needed to maintain media transparency.1 Evidence for this is seen in other conditions where vitreous liquefaction leads to premature cataract formation, such as degenerative myopia and Wagner-Stickler syndrome.1
The use of an oil or gas tamponade also significantly increases the rate of cataract formation. This may be due to intraoperative contact between the tamponade substance and the posterior lens capsule.1,3 Other factors may be excess glucose or oxygen in the BSS and excessive intraoperative light exposure which may cause oxidation of lens proteins.1
Choosing if and when to do cataract surgery after vitrectomy depends on several factors including the severity of cataract development, the risk of macular edema development, the severity of the underlying retinal disease and the visual potential of the eye.
When vitrectomy was first performed in the 1970s, it was standard practice to remove the crystalline lens concurrently since feathery posterior subcapsular cataracts (PSC) would often form intraoperatively. Once this was addressed by properly balancing infusion fluids, it was generally preferred to leave the lens in place for at least three months, if possible, as it was recognized that there was a higher prevalence of neovascular glaucoma (NVG) in phacovitrectomy patients who were diabetic.11 This occurred from unimpeded diffusion of vascular endothelial growth factor (VEGF) into the anterior chamber due to the lack of a barrier formed by the vitreous and lens. Once foldable IOLs were introduced, the incidence of NVG following phacovitrectomy declined to a reasonable rate in cases treated with PC IOL implants.3
In the present day, the most common circumstance where a combined procedure is unavoidable is when a lens opacity precludes adequate posterior segment views for vitreous dissection or treatment of underlying retinal disease.3,5 Beyond that, circumstances exist where a combined procedure may be optimal such as in cases of macular hole or ERM, where silicone oil must be used as a part of the retinal repair.
Silicone oil is used when a retinal tamponade is needed to repair a retinal detachment or to facilitate a membrane peel. It facilitates reattachment of the retina by displacing aqueous from the surface of the retina and disseminating between retinal folds and flattening them out.7,12 It can be lighter or heavier than aqueous depending on the specific chemical chosen, which enables it to accomplish the goal of forming a retinal tamponade in a variety of locations.12 Once the retina is firmly reattached, the silicone oil is usually removed, as it cannot be reabsorbed.7 Cataract surgery can be performed simultaneously with silicon oil extraction as a matter of expedience.1 However, calculating intraocular lens (IOL) power while silicone oil is present is difficult due to ultrasonography artifacts from the multiple fluid interfaces and variable amounts of silicone oil present. This is circumvented by calculating IOL power intraoperatively right after silicon oil removal.1
Silicone oil use is associated with posterior subcapsular cataract (PSC) formation and may accelerate the formation of visually significant cataracts.
Another circumstance where combined procedure may be preferred is when patients are over 50 years old and are deemed to be at high risk of imminent age-related cataract progression.1 This procedure is termed an “optional” phacovitrectomy as it is not medically indicated but may be overall preferable to hasten visual recovery.
Cataract extraction in post-vitrectomy patients presents its own variety of complications. These include smaller pupils, an extremely deep anterior chamber (which can affect the angle at which the instruments are held), increased mobility of the iris, increased mobility of the posterior capsule and zonular dehiscence. These complications increase risk of posterior capsule tears and loss of nuclear fragments posteriorly.1,4,5
The rate of posterior capsular opacification (PCO) in post-vitrectomy eyes is lowered when a combined PPV and CE procedure is performed in non-diabetic patients.13 For these reasons, surgeons may prefer performing a phacovitrectomy in such patients by default. However, this practice remains controversial as the literature has been equivocal on the benefit of combined versus separate procedures.1-14
A combined procedure is not recommended in those with diabetes. Phacovitrectomy in cases involving PDR has a greater incidence of PCO, anterior uveitis and posterior synechiae.5 In patients with vitreous hemorrhage, the red reflex may be degraded to the point where capsulorhexis becomes difficult, making a combined procedure more difficult.3 The presence of any iris neovascularization (NVI) is a clear contraindication for a combined procedure due to an increased risk of neovascular glaucoma.3 Diabetic patients also have a paradoxically lower rate of cataract development compared to non-diabetic vitrectomy cases and are at greater risk of retinal complications if they do undergo CE.14
If phacovitrecomy is not desired and the visual potential of the eye is limited by retinal pathology, it may be best to leave the cataract in place indefinitely. This may be the case in a severe tractional retinal detachment from PDR.3 Tests of macular function are helpful to determine the level of macular function when views of the retina are obscured by dense lens opacities. A retinometer/interferometer is an instrument that uses the principle of light interference to project a sharpened image onto the macula such that it bypasses any media opacities, allowing an evaluation of macular function. A potential acuity meter (PAM) can also estimate postoperative visual acuity by projecting a Snellen visual acuity chart via a very narrow beam of light through small “windows” in the opacities. The Purkinje tree can be used to subjectively test macular function in a way that bypasses media opacities. The test is performed by shining a light against the anterior sclera and asking the patient if they are able to distinguish the Purkinje tree created by the shadow of retinal blood vessels.
Ophthalmic ultrasound is also useful for detecting vitreous hemorrhage and retinal detachment when views of the posterior segment are totally obscured by lens opacities. In cases where CE is deferred indefinitely, it must be remembered that cataract progression can advance to the point of causing phacomorphic glaucoma or phacoanaphylaxis, which must be addressed immediately should they arise.
Outcome Assessment
Vitreoretinal surgical outcomes cannot be divorced from the outcome of post-vitrectomy cataract surgery. The decision of whether to perform cataract extraction at the time of the vitrectomy or to wait until a visually significant cataract develops depends on the type of vitreoretinal pathology of the patient and their perceived risk of imminent cataract development. The idea of combining cataract surgery with another surgical procedures is common. Patients who are left phakic following vitrectomy should be monitored regularly to address lens opacities promptly when they arise.
A combined procedure was not considered for this patient as she was diabetic. Phacovitrectomy may have increased the risk for PCO, anterior uveitis and posterior synechiae.
After cataract extraction, her vision OD improved to 20/80 postsurgically and she was pleased with her visual outcome. We advised her to wear full time polycarbonate spectacles to improve her function and protect her better-seeing eye.
Dr. Gurwood is a professor of clinical sciences at The Eye Institute of the Pennsylvania College of Optometry at Salus University. He is a co-chief of Primary Care Suite 3. He is attending medical staff in the department of ophthalmology at Albert Einstein Medical Center, Philadelphia. He has no financial interests to disclose.
|
1. Panozzo G, Parolini B. Cataracts associated with posterior segment surgery. Ophthalmol Clin North Am. 2004;17(4):557-vi. 2. Feng H, Adelman RA. Cataract formation following vitreoretinal procedures. Clin Ophthalmol. 2014;8:1957-65. 3. Lahey JM, Francis RR, Kearney JJ. Combining phacoemulsification with pars plana vitrectomy in patients with proliferative diabetic retinopathy: a series of 223 cases. Ophthalmology. 2003;110(7):1335-9. 4. Do DV, Gichuhi S, Vedula SS, Hawkins BS. Surgery for postvitrectomy cataract. Cochrane Database Syst Rev. 2018;1(1):CD006366. 5. Allen D, Steel DHW. Combined procedures. In: Yanoff M, Duker JS. Ophthalmology, 4th edition. Saunders: Elsevier, 2014;382-5. 6. Faulborn J, Conway BP, Machemer R. Surgical complications of pars plana vitreous surgery. Ophthalmology. 1978;85(2):116-25. 7. Holekamp NM, Shui YB, Beebe DC. Vitrectomy surgery increases oxygen exposure to the lens: a possible mechanism for nuclear cataract formation. Am J Ophthalmol. 2005;139(2):302-10. 8. Tornambe PE, Hilton GF, Brinton DA, et al. Pneumatic retinopexy. A two-year follow-up study of the multicenter clinical trial comparing pneumatic retinopexy with scleral buckling. Ophthalmology. 1991;98(7):1115-23. 9. Sebag J. Vitreous Anatomy and Pathology. In: Yanoff M, Duker JS. Ophthalmology, 4th edition. Saunders: Elsevier, 2014;430-6. 10. Engelbert M, Chang S. Vitrectomy. In: Yanoff M, Duker JS. Ophthalmology, 4th edition. Saunders: Elsevier, 2014;471-5. 11. Haimann MH, Abrams GW. Prevention of lens opacification during diabetic vitrectomy. Ophthalmology. 1984;91:116 -21. 12. Barca F, Caporossi T, Rizzo S. Silicone oil: different physical proprieties and clinical applications. BioMed Research International. 2014;Article ID 502143. 13. Roh JH, Sohn HJ, Lee DY, Shyn KH, Nam DH. Comparison of posterior capsular opacification between a combined procedure and a sequential procedure of pars plana vitrectomy and cataract surgery. Ophthalmologica. 2010;224(1):42-6. 14. Smiddy WE, Feuer W. Incidence of cataract extraction after diabetic vitrectomy. Retina. 2004;24(4):574-81. |

