Glaucoma Pearls & PitfallsIn the July 2024 issue of Review of Optometry, our 30th annual Glaucoma Report, seasoned ODs share tips on using modern tools, technology and knowledge to provide top-notch care to your patients with this chronic condition. Check out the other articles featured in this issue:
|
Over the last few decades, there have been many shifts in optometry’s approach to glaucoma diagnosis and management, resulting from an influx of new research and technology-driven knowledge. Below, we address several of the ongoing conversations that are allowing clinicians to develop a more up-to-date approach to glaucoma.
1. What is a “glaucoma suspect”?
One barrier to success in classification, risk identification and subsequent surveillance of our patients comes from a lack of clarity of the term “glaucoma suspect,” which is widely, but likely inconsistently, used in glaucoma nomenclature.1 Does family history—amongst a myriad of other risk factors—truly make someone a glaucoma suspect, or does accurate labeling of someone as a glaucoma suspect require signs consistent with actual glaucomatous optic nerve head damage, whether that be thinning of inferior- or superior-temporal rim and retinal nerve fiber tissue?
The authors of one recent editorial point out that the term “glaucoma suspect” is ambiguous and cannot be exclusively categorized as either a disease state or a risk factor.1 Broadly included under the umbrella of glaucoma suspect are both those with clinical findings suggestive of, but not yet definitive for, glaucomatous optic neuropathy, as well as those possessing traits and risk factors for glaucoma but lacking features even remotely suggestive of characteristic optic nerve head damage. The authors state that the vagueness of the term leads to incorrect diagnosis, errant treatment decisions and inappropriate follow-up intervals, confusion amongst providers and the resultant risk of negatively affecting the patient’s sense of wellbeing.
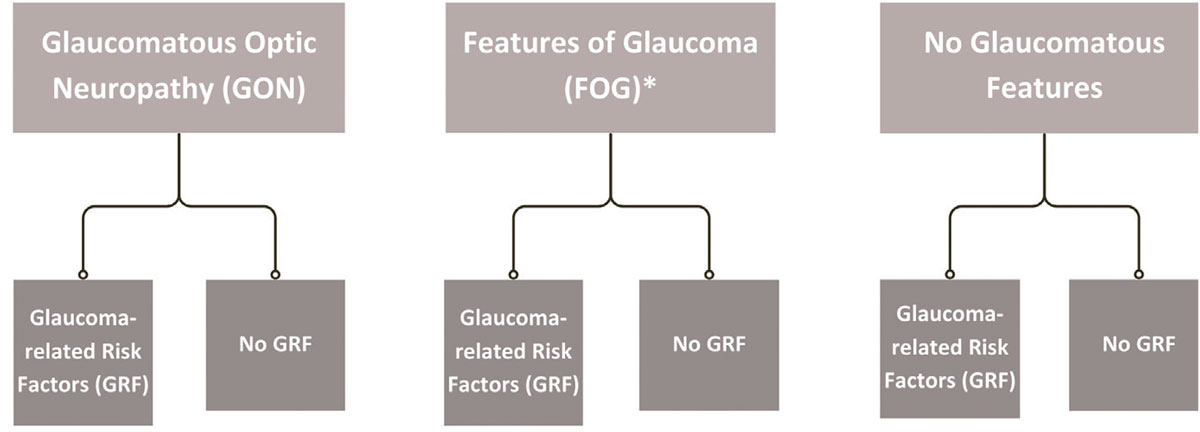
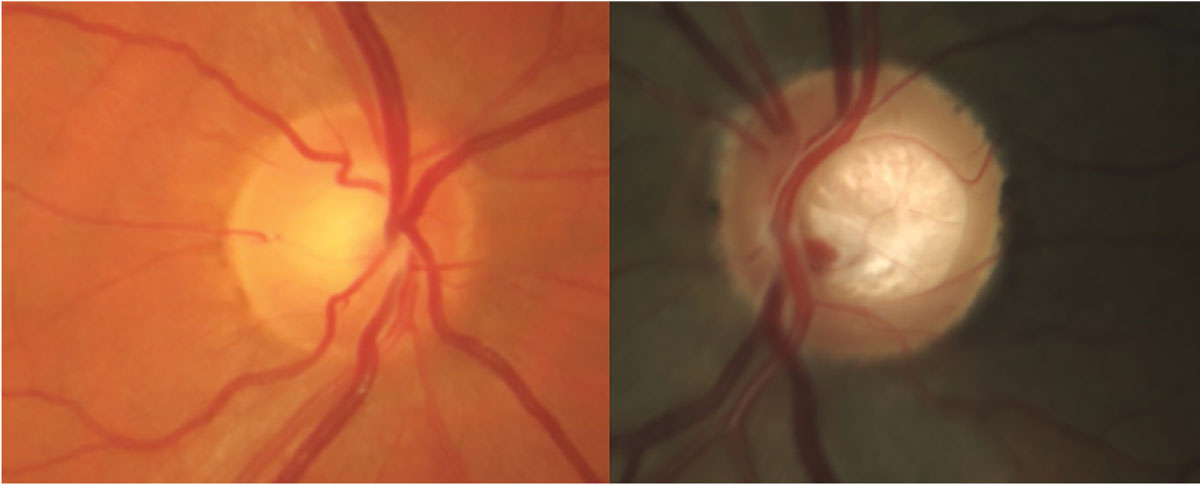
They propose abandonment of the term “glaucoma suspect” in favor of the more refined descriptions of such patients as having either features of glaucomatous optic neuropathy (FOG) and/or glaucoma-related risk factors (GRFs).1 This type of classification is illustrated by flowchart in Figure 1. Differentiating these two, as opposed to the current lumping of them together, is not a controversial subject, but rather one that provides an opportunity to define everyone’s overall condition, allowing for a more accurate reflection of the current optic nerve status and future risk to said status. Embracing the terms FOG and GRFs presents an opportunity to improve necessary care while reducing unnecessary care. Figure 2 illustrates two instances when these terms more aptly fit patients’ status.
 |
|
Fig. 1. Reclassification of glaucoma suspect into glaucomatous optic neuropathy, FOG (*includes pre-perimetric glaucoma) and no glaucoma, with further stratification of associated GRFs.1 Click image to enlarge. |
 |
|
Fig. 2. Two patients, both previously labeled as glaucoma suspects. On the left, this patient has IOPs of 26mm Hg OU, central corneal thickness of 523µm and positive family history. Ocular hypertension with GRFs would be a more appropriate classification. On the right, this patient displays laminar distension and questionably thin rim but no other risk factors. FOG with lack of GRFs more accurately depicts this patient’s status. Click image to enlarge. |
2. What are the limitations of tonometry?
As a reminder, intraocular pressure (IOP) is a GRF but not part of the definition of glaucoma. IOP has a wide distribution of normative values and therefore may vary per individual. The Ocular Hypertension Study demonstrated that a high IOP (≥24mm Hg) is a concern for increased risk of conversion to glaucoma, particularly when the corneal thickness is thinner than average.2 Focusing on a single IOP reading at an exam—or even three readings over the course of a year—concentrates attention on merely a snapshot of the patient’s true IOP range.
All tonometry methods, including the traditional gold standard, Goldmann applanation tonometry (GAT), have inaccuracies in measurement based on corneal thickness variations.3,4 IOP is also prone to diurnal/nocturnal variation. Furthermore, Valsava maneuvers, body position changes (heart elevated higher than head) and physical pressure on the eye can all increase IOP.5,6 This affects parameters used in clinical decision making, such as TMax (highest IOP without treatment) and treatment IOP target range, because research has shown patients with higher IOP peaks and wide IOP ranges are more likely to progress.7
Since the true IOP disparity is not observable in-office, 24-hour or continuous IOP devices have become available as a new evaluation modality.8 iCare Home was the first FDA-approved device in 2017 with portability and easy use for patients. The prescribing doctor is able to review the readings and make decisions based on the fluctuations, specifically at the highest peak reading. Unfortunately, this device does not take a continuous measurement, nor does it track IOP when the patient is sleeping. As with all tonometry methods, iCare is also influenced by corneal properties and does not always correspond with GAT.9
Triggerfish is a soft contact lens that takes a continuous 24-hour measurement, but it records changes in the corneoscleral junction curvature in millivolt equivalents (mV eq) rather than millimeters of mercury (mm Hg) as with IOP measurements. This variable might be superior to GAT for glaucoma monitoring but requires more research to verify its influence on risk of progression.10 Another advantage of a 24-hour device is that patients may be more inclined to use their glaucoma drops knowing their IOP is being measured outside the office.
If and when to use these remote monitoring devices is up to the prescribing doctor. If pursued, it would be wise to attain these measurements prior to treatment and/or as a tool to evaluate a treatment’s effect. Per the manufacturer website, iCare Home 2 must be prescribed and can be ordered via the company website; one-week rental of the device via a distributor costs around $250 and insurance may cover the product.
Another consideration when applying IOP as a GRF is that it is not the only force in play around the optic nerve head; both ocular perfusion pressure and cerebrospinal fluid pressure have been implicated in glaucoma but are less easily measurable.
3. How is corneal hysteresis relevant?
Traditionally, pachymetry has been considered the critical corneal metric to include in the baseline testing of glaucoma and ocular hypertensive patients. However, studies on corneal hysteresis (CH) over the last decade show that the relationship between the cornea and glaucoma involves more than just corneal thickness, as it is unlikely to indicate how the eye adapts to the multiple forces to which it is exposed.11,12 CH is a biomechanical property that reflects the cornea’s ability to absorb and release energy created by applanation forces during measurement. It has been suggested that the cornea’s ability to resist being deformed by applanation forces may provide a surrogate measure for the ability of the lamina cribrosa and peripapillary sclera to resist deformation from various confounding pressures.13 CH is now known to have a more significant association with the development of glaucoma as well as its risk and rate of progression.14 This association is considered to be much stronger than pachymetry and, therefore, CH is likely more valuable as a predictive factor.15
High CH values may confer a protective effect, whereas low CH values increase the eye’s susceptibility to glaucomatous damage. To highlight this point, a study comparing glaucoma patients with their non-glaucomatous counterparts reported an average CH of 8.95 ±1.27mm Hg in the disease group and 10.97 ±1.59mm Hg among controls.16 Similar findings have been replicated as more research on CH has been done.17 Subsequently, it may be time to universally include CH in the standard risk stratification and monitoring of glaucoma.18
In 2023, the American Academy of Ophthalmology’s Ophthalmic Technology Assessment Committee concluded that, although the interpretation of hysteresis is complex and no causal relationship with glaucoma has been proven, CH appears to be a potential adjunct in identifying disease risk, extent of disease and those at risk of progression and therefore should be considered complementary to structural and functional testing.17 Accordingly, CH has entered the mainstream and is accepted as a worthy component necessary to help solve the glaucoma puzzle. However, its application after diagnosis is not yet well understood.
4. Is there a vascular basis to glaucoma and, if so, do I need to start monitoring with OCT angiography?
Given the abundant blood supply necessary to perfuse the optic nerve head, the suspicion that there is a vascular basis to glaucoma is reasonable. Vascular dysregulation has been implicated in glaucoma research, especially in patients with low IOP.19 Optic disc hemorrhage, reduced mean and diastolic ocular perfusion pressure, isolated vasospastic conditions—such as migraines and Raynaud’s syndrome—vascular dysregulation found in Flammer syndrome and presence and enlargement of parapapillary atrophy in patients with glaucoma all suggest that vascular dysregulation plays a role in the pathogenesis of the disease.20-22
Multiple technologies have been used in research trying to measure blood flow in glaucoma, including laser speckle flowgraphy, intravenous fluorescein and indocyanine green angiography, laser Doppler flowmetry and retinal flowmetry, with some studies showing reduced optic nerve and peripapillary blood supply; others even demonstrate a correlation between the extent of blood flow disturbance and disease severity.21 Although each technology has contributed to understanding the role of vascular dysregulation on glaucoma, each has its own unique limitations and all have shown an inconsistent or lack of ability to obtain accurate, reproducible and quantitative information.23
OCT angiography (OCT-A) has emerged as a technology that provides non-invasive, high-quality imaging of the retinal and choroidal microvasculature that is reproducible and can provide quantitative data allowing for inter-visit comparisons.21,22 OCT-A studies over the last decade have consistently demonstrated a reduction in the peripapillary and both the para- and perifoveal capillary networks in patients with open-angle glaucoma compared with healthy controls. This reduced capillary density is in direct proportion to the severity of disease and its rate of progression. It also aligns with topographical structural loss and actually has a stronger correlation with functional testing than structural OCT measurements.22,24-27
Another benefit of OCT-A lies in cases where typical structural testing has reached its measurement floor due to severe stage or in eyes with high myopia not amenable to consistent machine segmentation. It also may be able to demonstrate medical or surgical success by confirming an increase in vessel density post-treatment and may even provide a marker of retinal ganglion cell dysfunction prior to cell death.28-31
A report by the American Academy of Ophthalmology concluded in 2021 that peripapillary, macular and choroidal vessel density parameters may complement functional and structural OCT measurements in the diagnosis of glaucoma.22 More recently, the authors of one study concluded that longitudinal OCT-A measurement complemented OCT structural measurements and, when combined, these measurements improved the accuracy of detecting visual field progression vs. either OCT-A or OCT alone.32 OCT-A has also shown to be repeatable for most platforms, which is necessary for any technology to be usable in monitoring disease progression. However, OCT-A tends to have longer scan acquisition time than OCT and therefore increased chance of fixation drift and motion artifact, which must be factored into analysis. Mahmoudinezhad et al. have recently shown that to detect progression, the average optimal OCT-A test frequency is two tests per year. This is the same number of tests per year that is recommended to minimize the time required to detect structural OCT.33
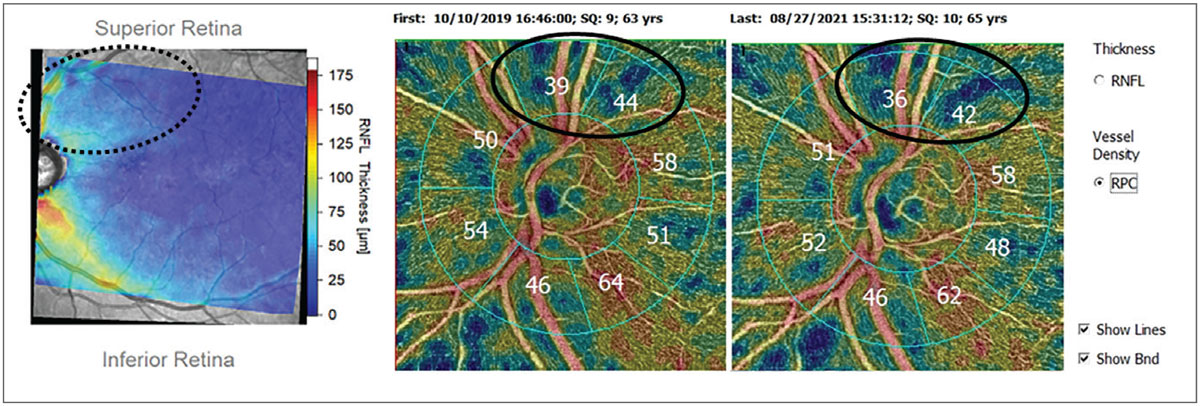
One of the major limitations in using OCT-A for monitoring disease is that not every platform has a substantial enough software package allowing for quantitative data to be measured and compared (Figure 3). Ultimately, the research shows that OCT-A can be a complementary piece in glaucoma diagnosis and management. As the technology becomes more widespread, how important OCT-A becomes in our glaucoma care remains to be seen.
 |
|
Fig. 3. Patient with glaucomatous damage to the superior arcuate bundle (dotted oval) shown on Hood thickness report (left). OCT-A of circumpapillary vessel density shows corresponding superior loss of vessel density (solid ovals) using OCT-A platform with analytics package and progression software. Click image to enlarge. |
5. What should be considered first-line therapy?
Topical glaucoma medications have been regarded as first-line treatment for glaucoma for decades. As of recently, selective laser trabeculoplasty (SLT) has been accepted as an appropriate first-line treatment as well. Unfamiliar procedures and devices may seem daunting to recommend but could be superior treatment in early cases.
Glaucoma drops not only require consistency of instillation but also disrupt the ocular surface, which leads to dry eye symptoms, thus contributing further to patient nonadherence. In addition, patients may have pre-existing dry eye or be on multiple glaucoma meds. In response, practitioners may switch to prescribing a different class, a more consistent brand or even preservative-free options. However, chronic use of the active drug is often the culprit of imbalance in ocular surface homeostasis.34 Recommending aggressive dry eye therapy may further impair patient adherence due to multiple treatments and increased costs, negatively affecting quality of life.35
To mitigate this, new drug delivery options and laser procedures offer excellent first-line treatment options with the understanding that drops or further procedures may still be necessary. The shift to a modern approach requires a fresh perspective and a different conversation with our patients.36
Clinicians’ hesitation to SLT as first-line therapy may stem from past approaches to treatment. SLT’s precursor, argon laser trabeculoplasty (ALT), was introduced in 1973 and was the predominant form of therapeutic glaucoma laser for decades until SLT received FDA approval in 2001.37 Since 2011, research has demonstrated strong evidence that SLT provides safe and effective 24-hour IOP control in patients with primary open-angle, juvenile open-angle, pigmentary and exfoliation forms of glaucoma as well as in patients with ocular hypertension.37
In spite of this effectiveness, medical therapy has remained the most common initial IOP-lowering intervention, with SLT often used as a supplement.38 Although practitioners have both advocated for and employed SLT over the last 20 years, there was lack of strong evidence that challenged medical therapy as first-line treatment until recently.
The SLT vs. Medical Therapy for Initial Treatment of Glaucoma Study and, more famously, the Laser in Glaucoma and Ocular Hypertension (LiGHT) trial found that SLT provided IOP control equivalent to medications, with both studies concluding SLT to be a safe and effective first-line treatment.39,40 Additionally, at the six-year mark of the LiGHT trial, patients who received SLT initially had less disease progression, required fewer incisional glaucoma surgeries and had quality-of-life metrics equivalent to patients initially started on drops.40 The evidence supporting SLT as first-line therapy has proved powerful enough that the UK’s National Institute for Health and Care Excellence has recently upgraded SLT to become its preferred first-line treatment. This sentiment has extended also to the European Glaucoma Society, which has updated its guidelines to include SLT as a first-line option. What’s more, a 2024 report by the American Academy of Ophthalmology’s Ophthalmic Technology Assessment Committee concluded that there is level 1 evidence substantiating SLT as an appropriate primary intervention strategy.37,41-42
Thus, recommending SLT as first-line to eligible patients has now become part of the standard informed consent process and is no longer considered controversial. Note that although in most trials SLT is as effective as topical therapy, SLT, like medication, is not effective on every eligible patient; when it is effective, that effect is not permanent and treatment may need repeating, as studies show treatment duration will vary from patient to patient.37,43
As optometrists become more involved in both recommending and performing SLT, we must continue to exert caution in our conversations with patients, clearly elucidating that SLT is not a cure but rather a tremendous drop-free, repeatable option to help slow the progression of their glaucoma.44,45 Furthermore, a retrospective study published in 2018 found that lower energy (0.4mJ/spot) 360˚ SLT when repeated annually had better outcomes than standard SLT settings applied on an as-needed basis.46 The ongoing Clarifying the Optimal Application of SLT Therapy trial is comparing this novel treatment approach to standard SLT application. When the results are published, they may dictate how often optometrists should perform or refer for this procedure.47
6. What are the sustained-release drug options?
With rising concern of the lower statistical likelihood that patients are both adherent to taking their drops and reliably instilling them, as well as the question of how much medication actually impacts the targeted mechanism inside the eye or bioavailability, comes the exploration of other therapeutic modalities. Sustained-release devices address the above concerns but can be limited to a niche category of patients who may respond well to ocular hypotensive medications but are not candidates for other avenues of therapy, such as laser trabeculoplasty or traditional incisional surgery like minimally invasive glaucoma surgery. This may simply be due to apprehensiveness about surgery, incompatible insurance coverage or costs regarding use of an ambulatory surgery center. The simplest approach to differentiating sustained-release devices is to distinguish by delivery location: ocular surface (i.e., adnexa, puncta) or intraocular (i.e., iridocorneal angle, trabecular meshwork).
Ocular surface. Sustained delivery to the ocular surface has been conceptualized as early as the inferior fornix-situated Ocusert (Alza Corporation) in 1975.48 The pilocarpine-impregnated elliptical plastic membrane proved that IOP could be controlled effectively without drops, but it required weekly replacements and was reported to cause unrelenting foreign body sensation. Conjunctival fornix–based medications have been explored as of 2024, including the preservative-free Bimatoprost Ocular Ring (Allergan) and the Topical Ophthalmic Drug Delivery Device (prostaglandin and timolol, Amorphex Therapeutics) with the latter lasting 90 days before needing replenishment and the former lasting six months with a slow decline in the amount of medication delivered, starting from 35µg/day and tapering off to only 6µg/day.49,50 Neither inserts have yet begun Phase III trials.49,50
Drug-eluting devices have also been explored in the puncta with Evolute (travoprost, Mati Therapeutics), the subconjunctival space via injection by Vi-Sci’s Eye-D (latanoprost, BioLight Life Sciences) and via soft contact lens delivery by LLT-MTT1 (bimatoprost, MediPrint Ophthalmics).51 Logistically, ocular surface-based eluting devices pose a myriad of questions about efficacy—will a patient feel an insert fall out? What would the cost be to replace it for the patient? What material works best for eluting medication? How will this impact patients who already have ocular surface disease?
 |
|
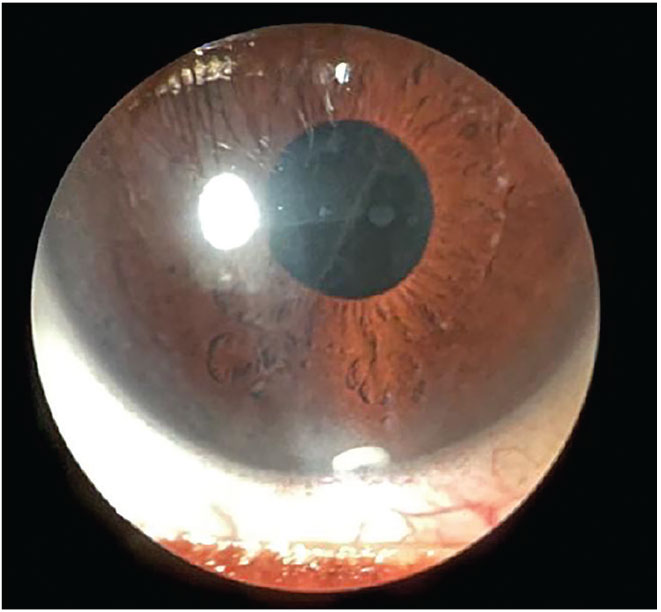
Fig. 4. Durysta sustained-release implant visible in inferior anterior chamber three months post-injection. Click image to enlarge. |
Intraocular. The intraocular sustained-release devices may be superior in reliability, adherence and consistency. These medications are delivered directly into the eye, thus removing reliance on patient adherence and accuracy of alternate ocular surface therapies. To date, there have been several iridocorneal injectables: Durysta (bimatoprost, Allergan), OTX-TIC (latanoprost, Ocular Therapeutix, Phase II) and the ENV515 (latanoprost, Envisia Therapeutics, Phase II). Durysta is currently the only FDA-approved sustained-release therapy that can be implanted in-office; however, this will depend on the comfort level of the physician and appropriate scope of practice laws (Figure 4).52 Durysta offers a 30% reduction in IOP for the course of three to four months for the average patient, with some patients benefiting much longer. However, Durysta is currently FDA-approved for a single insertion within the lifetime of the patient.
In clinical experience, patients tolerate the procedure well with minimal inferior circumlimbal injection as the pellet settles inferiorly. However, providers should be aware that the pellet presence can affect corneal endothelial cell count (ARTEMIS-1 showed a 10.2% incidence of ≥20% endothelial cell density loss with a 10µg implant) and can potentially maneuver its way to the posterior chamber in patients with compromised or absent lens capsules.53 The biggest hurdle patients and physicians will face is that Durysta is FDA-approved (and reimbursed by insurance) for just a single administration to each eye. Therefore, surgeons will have to transfer the full cost to the patient if a patient succeeds with Durysta and requests another months or years later.
Last to consider is the trabecular meshwork implant iDose TR (travoprost, Glaukos).54 The intracameral iDose elutes 75mcg of the drug from a titanium implant that is inserted through the trabecular meshwork and anchored to the sclera, eluting travoprost for several months up to three years per current FDA trials comparing 12 month data. It is contraindicated in those with Fuchs’ endothelial dystrophy or with history of any corneal transplant but does boast a robust safety profile with minimal adverse effects per Glaukos. The device is visible upon slit lamp exam and much like other injectables, it can migrate or become dislodged. Thus, it is imperative to check patients at regular intervals.55 It is expected to launch this year, with a single implant costing $13,950.
7. To LPI or not to LPI?
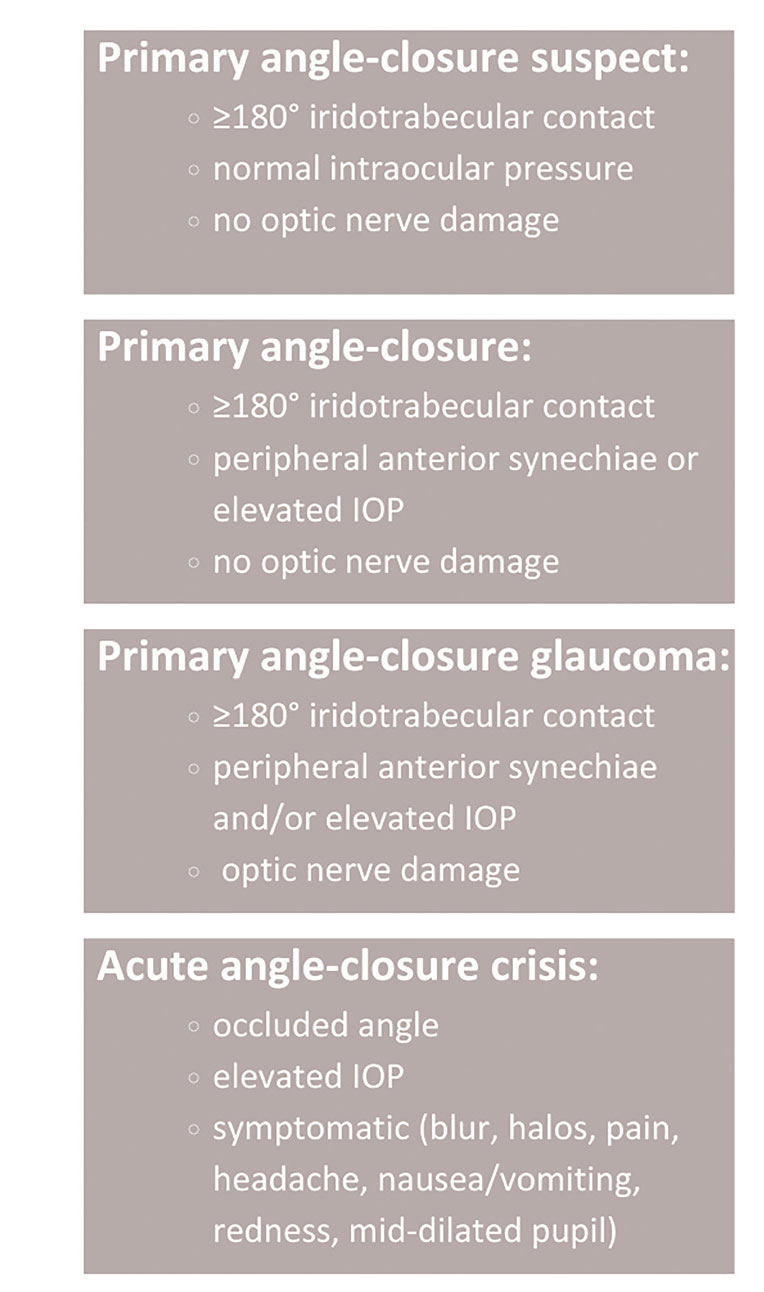
What is the best approach to managing primary angle-closure suspects? Figure 5 delineates these patients into primary angle closure, primary angle-closure glaucoma and acute angle-closure crisis categories.56 The approach is contingent on accurate assessment of the angle along with risk factor analysis.
Gonioscopy remains the accepted method for visibly evaluating the structures of the anterior chamber angle and the interaction between those structures and the iris. Unfortunately, per surveys and retrospective research, gonioscopy is the most underused test in a glaucoma risk assessment.57,58 Without it, the common diagnosis of primary open angle glaucoma cannot be assumed based on Van Herick angle estimation and IOP expectations. It is critical to understand that angle closure can not only be acute or chronic but also may or may not be associated with elevated IOP or glaucomatous damage.59
 |
|
Fig. 5. Primary angle closure: “Narrow angle” is a vague term and applied inconsistently among physicians. The nomenclature at left is extracted from American Academy of Ophthalmology Preferred Practice Patterns.55 Click image to enlarge. |
While the standard of care for an acute angle-closure crisis (AACC) has not changed (topical/oral aqueous suppressants to lower IOP followed by an iridotomy), the protocol for primary angle-closure suspects is less standardized. The management approach also varies widely among eyecare practitioners. Based on risk vs. benefits, laser peripheral iridotomy (LPI) was historically favored over observation. The confusion exists because of the inability to predict likelihood of AACC. The Zhongshan Angle-closure Prevention 14-year trial demonstrated that incidence of primary angle closure was three times lower after LPI—primarily a lower risk of synechiae formation, which may be of little clinical significance.60
The researchers concluded, however, that prophylactic LPI for primary angle closure is not recommended, as the long-term risk of progression is still low at 1.4% per eye per year.60 An additional consideration is that participants were of Asian descent, and this ethnicity has an even higher risk of developing angle-closure crisis or glaucoma compared with other ethnicities.60
Multiple observational studies indicate that primary angle-closure suspects without increased IOP or posterior synechiae rarely develop acute angle-closure or chronic glaucoma. Low-risk patients can be monitored closely without laser intervention. If choosing to monitor, the patient should be warned of AACC symptoms—sudden blur, halos around lights, eye pain, periorbital headache, nausea and eye redness—so that they seek immediate care.
Patients that may benefit from a prophylactic LPI include those with AACC in the contralateral eye or anticholinergic/adrenergic medications that may induce a pupillary block. Other common risk factors include Asian or Inuit descent, hyperopia or short axial length and cataract progression. The patient’s health status, location and occupation can each complicate the process of seeking urgent ophthalmic care, which can also influence the decision.
Despite potentially superfluous procedures, the complications of LPI are minimal.59 Vertical dysphotopsias (i.e., glare, halos, lines, ghosting) have a low incidence rate of 2% to 3%, theorized to be caused by light scatter from the upper eyelid and tear film bisecting the iridotomy opening.61 However, current literature does not show a significant relationship between iridotomy location and dysphotopsia rates.61-63
Per the EAGLE study, removal of the lens via cataract surgery or clear lens extraction lowered IOP more effectively (mean IOP 1mm Hg difference) than LPI in patients with primary angle closure or primary angle-closure glaucoma. Phacoemulsification leads to widening of the anterior chamber and reduction of IOP, with similar or even superior outcomes compared with LPI. This approach should be highly considered for patients that are eligible for cataract surgery.64,65
Gonioscopy EssentialsThis procedure requires a dark room with a shortened light beam to avoid touching the pupil to minimize pupillary constriction and simulate the naturally dilated state of the iris and pupil.
|
8. What are the benefits vs. limitations for virtual reality visual field testers?
Glaucoma management requires both observation of the structural and functional changes. Technology has advanced the ability to detect structural change, yet functional testing has changed very little. Automated perimetry enables physicians to perceive glaucoma progression but has always been critiqued for the large size of the machine, the learning curves for administering and performing perimetry by staff and patient alike—and of course the biggest hurdle—the reliability of the patient during the test.
There has always been a desire to better map the functional changes in optic neuropathies with more ease. So-called virtual reality visual field (VRVF) instruments are portable, chargeable devices that can be used for patients who may not physically fit in a traditional visual field due to disability, wheelchair or being bedridden. A list of ones available can be found in Table 1. The majority possess glaucoma threshold capability and present visual stimuli in the same visual positions as standard automated perimetry (SAP).66 The ability for the target and field to move with patient eye movement allows for less patient error when compared with static fields that produce high false positives when the patient cannot help but search for the stimulus, even when instructed against it.67,68
 |
| Click image to enlarge. |
These head-mounted, gaze-tracking devices accomplish the basic needs of mapping a visual field. VRVFs also have a lower cost compared to purchasing a new or even used Humphrey Field Analyzer. Certain devices, such as the VirtualEye, will even perform pupillometry and color vision testing, which can streamline the patient’s screening/workup. The VisuAll (Olleyes) employs a unique pediatric, game-like strategy to keep engagement of the patient.69 In an attempt to detect early functional loss, nGoggle has been exploring visual evoked potential, which provides an objective map of functional loss.70,71
Per manufacturer websites, most VRVFs are also capable of presenting information in other languages, which can improve the reliability and inclusivity for non-English speaking patients. Cost is of course a factor as well. Many companies offer monthly subscription-based plans.
Despite the future of VRVFs being promising, there are several factors left to consider. Each company provides its own algorithms for threshold testing, which are based on the heavily studied and performed SAP. Although most variables, such as stimulus size, are comparable, some programs do not possess the same standards, such as different types of luminance.
There are different biases among each VRVF reference database; do they compare to the databases we are all familiar with? There is also lack of progression analysis with several VRVFs, which is considered essential to check for stability over time, as well as lack of generated structure-function overlay reports such as the PanoMap (Cirrus HD-OCT, Zeiss) or GMPE Hood Glaucoma Report (Heidelberg Spectralis, Heidelberg Engineering).
As time progresses and data becomes more established, the ability of these tests to stretch beyond in-person testing could be considerable. Home-based testing is becoming more prevalent in various aspects of healthcare; blood pressure and blood glucose monitoring allow the patient to be more mindful and present in their health. Why should ocular disease be any different?72 The advent of home-monitoring is enticing, but the same pressures to perform the test reliably must be considered. Regardless, visual field progression can be detected much more quickly and efficiently, hastening an office visit for more aggressive treatment.
9. Is the transition to SITA FASTER seamless?
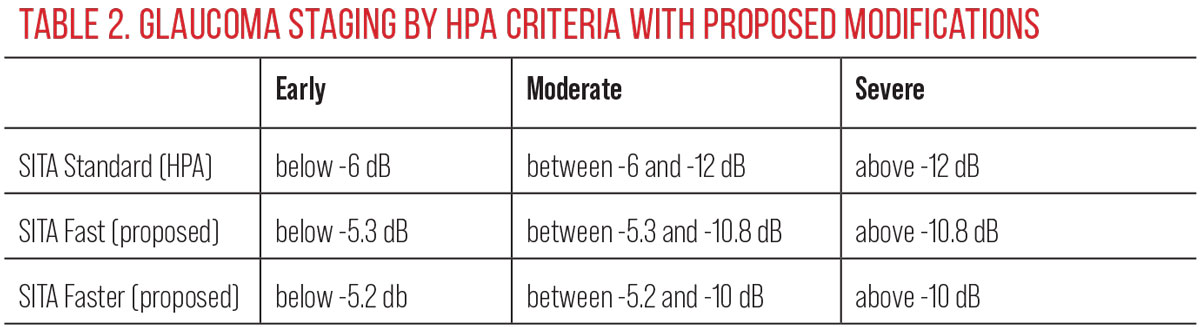
Released in 2019, the SITA Faster testing strategy was an important addition. This newer development reduces patient test burden over the original SITA test by cutting testing time by close to 60% as well as providing denser sampling of potentially damaged retinal ganglion cells with the 24-2C grid. Accordingly, transitioning from the original SITA Standard to SITA Fast and now to SITA Faster when doctors upgrade their software seems sensible. SITA Faster is not, however, infallible—studies show a 30% to 49% unreliable rate compared with a smaller 10.8% to 16.6% rate with SITA Standard, caused by initial low sensitivity measurements and relatively more severe global indices.73
Luckily, SITA Standard and SITA Faster have similar test-retest variability, comparable sensitivity and specificity and overall agreement amongst most field parameters.73 Pham et al. in 2021 showed transition from SITA Standard to SITA Faster showed similar mean deviation results in mild stage disease and in suspects but problematically resulted in higher mean deviation in moderate and severe stage disease, possibly masking disease progression.74 Recent research from the same group showed also that in the transition, applying traditional SITA Standard criterion (Hodapp-Parrish-Anderson criterion) to SITA Faster test data can result in an artificially “better” result and subsequent misclassification of disease severity and misdiagnosis of the rate of disease progression.75
These researchers have proposed that when assessing tests using SITA Fast and SITA Faster strategies, modified criteria would better reflect disease severity and assessment of the rate of progression (Table 2). From a practitioner standpoint, more accurate information provides a more accurate perception of the disease state and helps drive timely intervention.75 As we transition to newer strategies—even if they are on the same platform—we need to be vigilant in our practices in attaining sufficient information to determine change once we transition strategies rather than assuming older data will blend perfectly with the new.
 |
| Click image to enlarge. |
Takeaways
GRFs play a major role in predicting whether our patients will develop disease and consequently how they may progress. However, this does not automatically mean a patient has or will get glaucoma, changing how we use the term “glaucoma suspect.” While IOP is currently the only modifiable risk factor, the dynamics of tonometry, the value of CH and the potential of vascular etiology should also be considered.
Exhausting all topical treatment options before suggesting a procedure no longer serves our glaucoma patients. Recent research demonstrates the benefits of SLT and sustained-release drugs as first-line options, but communicating these options effectively is needed to align with modern care. With better understanding of primary angle closure, there has been less peripheral iridotomy referrals which will continue to decrease as practitioners improve gonioscopy skills and consider monitoring closely or cataract extraction. Lastly, understanding updates to visual field testing algorithms and innovative virtual reality fields will allow doctors to better care for their patients.
Dr. Ragha is an assistant professor at Southern College of Optometry and works in the affiliated clinics, The Eye Center and The Focal Point, in the ocular disease and primary care departments. She is a fellow of the American Academy of Optometry. Dr. Rixon is an attending optometrist at the Memphis Veteran Affairs Medical Center (VAMC). He is a diplomate in glaucoma through the American Academy of Optometry and member of the Optometric Glaucoma Society. Dr. Kirk joined VRF Eye Specialty Group in Memphis and has been performing surgical consultations and management for refractive, cornea, cataract and glaucoma surgery. They have no financial interests to disclose.
1. Leshno A, Shukla AG, Liebmann JM. Is it time to revisit glaucoma suspect nomenclature? Ophthalmol Glaucoma. 2024;7(3):219-21. 2. Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):714-20. 3. Tonnu P-A, Ho T, Sharma K, et al. A comparison of four methods of tonometry: method agreement and interobserver variability. Br J Ophthalmol. 2005;89(7):847-50. 4. Galgauskas S, Strupaite R, Strelkauskaite E, Asoklis R. Comparison of intraocular pressure measurements with different contact tonometers in young healthy persons. Int J Ophthalmol. 2016;9(1):76-80. 5. Rafuse PE, Mills DW, Hooper PL, Chang TS, Wolf R. Effects of Valsalva’s manoeuvre on intraocular pressure. Can J Ophthalmol. 1994;29(2):73-6. 6. Rüfer F. [Sources of error in Goldmann applanation tonometry]. Ophthalmologe. 2011;108(6):546-52. 7. Hughes E, Spry P, Diamond J. 24-hour monitoring of intraocular pressure in glaucoma management: a retrospective review. J Glaucoma. 2003;12(3):232-6. 8. Ittoop SM, SooHoo JR, Seibold LK, Mansouri K, Kahook MY. Systematic review of current devices for 24-h intraocular pressure monitoring. Adv Ther. 2016;33(10):1679-90. 9. Takagi D, Sawada A, Yamamoto T. Evaluation of a new rebound self-tonometer, Icare HOME: comparison with Goldmann applanation tonometer. J Glaucoma. 2017;26(7):613-8. 10. De Moraes CG, Mansouri K, Liebmann JM, Ritch R, for the Triggerfish Consortium. Association between 24-hour intraocular pressure monitored with contact lens sensor and visual field progression in older adults with glaucoma. JAMA Ophthalmol. 2018;136(7):779-85. 11. Sng CCA, Ang M, Barton K. Central corneal thickness in glaucoma. Curr Opin Ophthalmol. 2017;28(2):120-6. 12. Deol M, Taylor DA, Radcliffe NM. Corneal hysteresis and its relevance to glaucoma. Curr Opin Ophthalmol. 2015;26(2):96-102. 13. Wells AP, Garway-Heath DF, Poostchi A, et al. Corneal hysteresis but not corneal thickness correlates with optic nerve surface compliance in glaucoma patients. Invest Ophthalmol Vis Sci. 2008;49(8):3262-8. 14. Mohammadzadeh V, Moghimi S, Nishida T, et al. Effect of corneal hysteresis on the rates of microvasculature loss in glaucoma. Ophthalmol Glaucoma. 2023;6(2):177-86. 15. Jammal AA, Medeiros FA. Corneal hysteresis: ready for prime time? Curr Opin Ophthalmol. 2022;33(3):243-9. 16. Mangouritsas G, Morphis G, Mourtzoukos S, Feretis E. Association between corneal hysteresis and central corneal thickness in glaucomatous and non-glaucomatous eyes. Acta Ophthalmol. 2009;87(8):901-5. 17. Sit AJ, Chen TC, Takusagawa HL, et al. Corneal hysteresis for the diagnosis of glaucoma and assessment of progression risk: a report by the American Academy of Ophthalmology. Ophthalmology. 2023;130(4):433-42. 18. Murtagh P, O’Brien C. Corneal hysteresis, intraocular pressure, and progression of glaucoma: time for a “hyst-oric” change in clinical practice? J Clin Med. 2022;11(10):2895. 19. Weinreb RN, Harris A. 6th consensus meeting: ocular blood flow in glaucoma. Kugler Publications, 2009. 20. Flammer J, Konieczka K. The discovery of the Flammer syndrome: a historical and personal perspective. EPMA J. 2017;8(2):75-97. 21. Daneshvar R, Nouri-Mahdavi K. Optical coherence tomography angiography: a new tool in glaucoma diagnostics and research. J Ophthalmic Vis Res. 2017;12(3):325-32. 22. WuDunn D, Takusagawa HL, Sit AJ, et al. OCT angiography for the diagnosis of glaucoma: a report by the American Academy of Ophthalmology. Ophthalmology. 2021;128(8):1222-35. 23. Rao HL, Pradhan ZS, Suh MH, et al. Optical coherence tomography angiography in glaucoma. J Glaucoma. 2020;29(4):312-21. 24. Yarmohammadi A, Zangwill LM, Diniz-Filho A, et al. Relationship between optical coherence tomography angiography vessel density and severity of visual field loss in glaucoma. Ophthalmology. 2016;123(12):2498-508. 25. Lee A, Kim KE, Song WK, Yoon J, Kook MS. Progressive macular vessel density loss and visual field progression in open-angle glaucoma eyes with central visual field damage. Ophthalmol Glaucoma. 2024;7(1):16-29. 26. Shin JW, Song MK, Kook MS. Association between progressive retinal capillary density loss and visual field progression in open-angle glaucoma patients according to disease stage. Am J Ophthalmol. 2021;226:137-47. 27. Wu J-H, Moghimi S, Nishida T, et al. Association of macular vessel density and ganglion cell complex thickness with central visual field progression in glaucoma. Br J Ophthalmol. 2023;107(12):1828-33. 28. Moghimi S, Bowd C, Zangwill LM, et al. Measurement floors and dynamic ranges of OCT and OCT angiography in glaucoma. Ophthalmology. 2019;126(7):980-8. 29. Kwon HJ, Kwon J, Sung KR. Additive role of optical coherence tomography angiography vessel density measurements in glaucoma diagnoses. Korean J Ophthalmol. 2019;33(4):315-25. 30. Fry LE, Fahy E, Chrysostomou V, et al. The coma in glaucoma: retinal ganglion cell dysfunction and recovery. Prog Retin Eye Res. 2018;65:77-92. 31. Kim J-A, Kim T-W, Lee EJ, Girard MJA, Mari JM. Microvascular changes in peripapillary and optic nerve head tissues after trabeculectomy in primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2018;59(11):4614-21. 32. Kamalipour A, Moghimi S, Khosravi P, et al. Combining optical coherence tomography and optical coherence tomography angiography longitudinal data for the detection of visual field progression in glaucoma. Am J Ophthalmol. 2023;246:141-54. 33. Mahmoudinezhad G, Moghimi S, Proudfoot JA, et al. Effect of testing frequency on the time to detect glaucoma progression with optical coherence tomography (OCT) and OCT angiography. Am J Ophthalmol. 2023;245:184-92. 34. Holló G, Katsanos A, Boboridis KG, Irkec M, Konstas AGP. Preservative-free prostaglandin analogs and prostaglandin/timolol fixed combinations in the treatment of glaucoma: efficacy, safety and potential advantages. Drugs. 2018;78(1):39-64. 35. Skalicky SE, Goldberg I, McCluskey P. Ocular surface disease and quality of life in patients with glaucoma. Am J Ophthalmol. 2012;153(1):1-9.e2. 36. Radcliffe NM, Shah M, Samuelson TW. Challenging the ‘‘topical medications-first’’ approach to glaucoma: a treatment paradigm in evolution. Ophthalmol Ther. 2023;12(6):2823-9. 37. Takusagawa HL, Hoguet A, Sit AJ, et al. Selective laser trabeculoplasty for the treatment of glaucoma: a report by the American Academy of Ophthalmology. Ophthalmology. 2024;131(1):37-47. 38. Gedde SJ, Vinod K, Wright MM, et al.; American Academy of Ophthalmology Preferred Practice Pattern Glaucoma Committee. Primary open-angle glaucoma preferred practice pattern. Ophthalmology. 2021;128(1):P71-150. 39. Katz LJ, Steinmann WC, Kabir A, et al.; SLT/Med Study Group. Selective laser trabeculoplasty vs. medical therapy as initial treatment of glaucoma: a prospective, randomized trial. J Glaucoma. 2012;21(7):460-8. 40. Gazzard G, Konstantakopoulou E, Garway-Heath D, et al.; LiGHT Trial Study Group. Selective laser trabeculoplasty vs. eye drops for first-line treatment of ocular hypertension and glaucoma (LiGHT): a multicentre randomised controlled trial. Lancet. 2019;393(10180):1505-16. 41. Glaucoma: diagnosis and management. National Institute for Health and Care Excellence. www.nice.org.uk/guidance/ng81. Last updated January 26, 2022. Accessed April 22, 2024. 42. European Glaucoma Society terminology and guidelines for glaucoma, 5th edition. Br J Ophthalmol. 2021;105(Suppl 1):1-169. 43. Bedrood S, Berdahl J, Sheybani A, Singh IP. Alternatives to topical glaucoma medication for glaucoma management. Clin Ophthalmol. 2023;17:3899-913. 44. Hussain ZS, Muayad J, Harvey BJ, et al. Rates of laser trabeculoplasty by ophthalmologists and optometrists: a comparative analysis of the CMS Medicare public use file. Clin Ophthalmol. 2024;18:269-75. 45. Konstantakopoulou E, Jones L, Nathwani N, Gazzard G. Selective laser trabeculoplasty (SLT) performed by optometrists—enablers and barriers to a shift in service delivery. Eye (Lond). 2022;36(10):2006-12. 46. Gandolfi S. Low power selective laser trabeculoplasty (SLT) repeated yearly as primary treatment in open angle glaucoma(s): long term comparison with conventional SLT and ALT. E-Abstract 3459. Association for Research in Vision and Ophthalmology Annual Meeting; May 1, 2018. 47. Realini T, Gazzard G, Latina M, Kass M. Low-energy selective laser trabeculoplasty repeated annually: rationale for the COAST trial. J Glaucoma. 2021;30(7):545-51. 48. Mariz M, Murta J, Gil MH, Ferreira P. An ocular insert with zero-order extended delivery: release kinetics and mathematical models. Eur J Pharm Biopharm. 2022;181:79-87. 49. Brandt JD, DuBiner HB, Benza R, et al.; Collaborators. Long-term safety and efficacy of a sustained-release bimatoprost ocular ring. Ophthalmology. 2017;124(10):1565-6. 50. Leahy CD, Gutner R, Varney W, et al. Continuous wear non-invasive device for sustained ocular drug delivery. Invest Ophthalmol Vis Sci. 2014;55:481. 51. Singh RB, Ichhpujani P, Thakur S, Jindal S. Promising therapeutic drug delivery systems for glaucoma: a comprehensive review. Ther Adv Ophthalmol. 2020;12:2515841420905740. 52. Teymoorian S, Craven ER, Nguyen L, Werts E. Real-world study of the effectiveness and safety of intracameral bimatoprost implant in a clinical setting in the United States. Clin Ophthalmol. 2024;18:187-99. 53. Medeiros FA, Walters TR, Kolko M, et al.; ARTEMIS 1 Study Group. Phase 3, randomized, 20-month study of bimatoprost implant in open-angle glaucoma and ocular hypertension (ARTEMIS 1). Ophthalmology. 2020;127(12):1627-41. 54. Katz LJ, Sarkisian SR, Voskanyan LA, et al. Results of the prospective, randomized, controlled, multicenter phase 3 trials of the travoprost intraocular implant vs. topical timolol. Invest Ophthalmol Vis Sci. 2023;64(8):4296. 55. Ibach M. Interim results of a prospective phase II study of travoprost intraocular implants. Paper presented at: the American Academy of Optometry Annual Meeting; November 9, 2018; San Antonio, Texas. 56. Gedde SJ, Chen PP, Muir KW, et al. Primary angle-closure disease preferred practice pattern. Ophthalmology. 2021;128(1):P30-70. 57. Coleman AL, Yu F, Evans SJ. Use of gonioscopy in medicare beneficiaries before glaucoma surgery. J Glaucoma. 2006;15(6):486-93. 58. Hertzog LH, Albrecht KG, LaBree L, Lee PP. Glaucoma care and conformance with preferred practice patterns. Examination of the private, community-based ophthalmologist. Ophthalmology. 1996;103(7):1009-13. 59. Oh WH, Kim BG, Kyung H, Lee JH. Primary angle-closure glaucoma with normal intraocular pressure at the first visit: its prevalence and ocular characteristics. J Glaucoma. 2019;28(1):32-7. 60. Yuan Y, Wang W, Xiong R, et al. Fourteen-year outcome of angle-closure prevention with laser iridotomy in the Zhongshan Angle-Closure Prevention Study: extended follow-up of a randomized controlled trial. Ophthalmology. 2023;130(8):786-94. 61. Balas M, Mathew DJ. Dysphotopsia and location of laser iridotomy: a systematic review. Eye (Lond). 2024;38(7):1240-5. 62. Kavitha S, Ramulu P, Venkatesh R, et al. Resolution of visual dysphotopsias after laser iridotomy: six-month follow-up. Ophthalmology. 2019;126(3):469-71. 63. Srinivasan K, Zebardast N, Krishnamurthy P, et al. Comparison of new visual disturbances after superior vs. nasal/temporal laser peripheral iridotomy: a prospective randomized trial. Ophthalmology. 2018;125(3):345-51. 64. Azuara-Blanco A, Burr J, Ramsay C, et al.; EAGLE Study Group. Effectiveness of early lens extraction for the treatment of primary angle-closure glaucoma (EAGLE): a randomized controlled trial. Lancet. 2016;388(10052):1389-97. 65. Sharif RK, Aljahdali FF, Aljabri HM, et al. Phacoemulsification vs. laser peripheral iridotomy for treating primary angle closure glaucoma: a systematic review and meta-analysis. Clin Ophthalmol. 2024;18:1023-32. 66. Mees L, Upadhyaya S, Kumar P, et al. Validation of a head-mounted virtual reality visual field screening device. J Glaucoma. 2020;29(2):86-91. 67. Adhanom IB, MacNeilage P, Folmer E. Eye tracking in virtual reality: a broad review of applications and challenges. Virtual Real. 2023;27(2):1481-505. 68. Kong YXG. Visual field testing in the era of portable consumer technology. Clin Exp Ophthalmol. 2018;46(4):325-6. 69. Alvarez-Falcón S, Wang B, Taleb E, et al. Performance of VisuALL virtual reality visual field testing in healthy children. J AAPOS. 2024;28(1):103802. 70. Hood DC, Greenstein VC. Multifocal VEP and ganglion cell damage: applications and limitations for the study of glaucoma. Prog Retin Eye Res. 2003;22(2):201-51. 71. Nakanishi M, Wang Y-T, Jung T-P, et al. Detecting glaucoma with a portable brain-computer interface for objective assessment of visual function loss. JAMA Ophthalmol. 2017;135(6):550-7. 72. McLaughlin DE, Savatovsky EJ, O’Brien RC, et al. Reliability of visual field testing in a telehealth setting using a head-mounted device: a pilot study. J Glaucoma. 2024;33(1):15-23. 73. Lee GA, Kong GYX, Liu C-H. Visual fields in glaucoma: Where are we now? Clin Experiment Ophthalmol. 2023;51(2):162‐9. 74. Pham AT, Ramulu PY, Boland MV, Yohannan J. The effect of transitioning from SITA Standard to SITA Faster on visual field performance. Ophthalmology. 2021;128(10):1417-25. 75. Bradley C, Almidani L, Herbert P, Yohannan J. Estimating percent misdiagnosis when applying SITA-Standard criteria to SITA-Fast and SITA-Faster. ARVO 2024 annual meeting. |

