Eyelids & Ocular SurfaceThe November 2024 Review of Optometry issue showcases five articles on eyelid & ocular surface conditions, from MGD to scleritis. Read about what to do when dry eye patients aren’t responding to treatment, corneal AEs caused by systemic meds, innovative MGD treatments, managing ocular cellulitis and more. Check out the other articles featured in this issue: |
It should come as no surprise that meibomian gland dysfunction (MGD) is the chief cause of evaporative dry eye disease (DED), as inflammation and blockage of the glands lead to inadequate lipid secretion. This deficiency destabilizes the tear film and increases tear evaporation. So, our efforts to improve the lives of dry sufferers nearly always involve restoration of meibomian gland function. The good news is, there are a lot of treatments available. The bad news is—you guessed it—there are a lot of treatments available.
Optometrists need to stay up to date on a wide array of pharmaceutical and procedural MGD interventions, and the list continues to grow every year. Not every practice will be set up to offer everything, but we should all be mindful of the treatment landscape so that we can make informed choices about any new options we want to consider. Let’s explore what’s being offered so you have a better understanding of your options and how many of the newest ones continue to target the pathophysiology of MGD directly for more long-lasting results.
Thermal Treatments
In patients with MGD, the meibum tends to be thicker and more stagnant, and has a higher melting point compared to that of a healthy individual. Applying heat to the eyelids can help liquefy the altered meibum, promoting better secretion. Although not new, thermal treatments like LipiFlow (Johnson & Johnson) and iLux (Alcon) remain helpful in treating obstructive MGD. These procedures apply localized heat to the inner eyelids and gentle pressure to release blocked meibomian glands, while other devices like TearCare (Sight Sciences) and MiBoFlo (MiBo Medical Group) apply heat on the external lids and require manual meibomian gland expression (MGE) (Figure 1). Pairing this approach with microblepharoexfoliation, which removes biofilm and debris from the lid margins, can enhance the efficacy of gland evacuation during thermal pulsation or MGE.
Recently, radiofrequency treatment has been advocated for MGD; it works by liquefying meibum and clearing blocked glands. A radiofrequency device releases an electromagnetic wave that converts energy from an oscillating electric field into heat by vibrating charged particles. It can be used as a standalone treatment; however, most evidence supporting its efficacy comes from studies that examine its use in conjunction with intense pulsed light (IPL).1
There is an increasing body of evidence supporting the use of IPL, a technology that uses a flash lamp to generate a spectrum of polychromatic light ranging from 500nm to 1200nm, which is filtered to deliver selective photothermal effects on specific tissues. IPL targets the abnormal blood vessels contributing to inflammation around the eyelids and has been shown to be successful in reducing vascularity and inflammation, as well as improving tear film quality.2,3 Clinical trials indicate that combining IPL with MGE yields better results than MGE alone.2,4,5 The best candidates for IPL are those with facial or ocular rosacea and Fitzpatrick skin types I to IV.
While MGD affects several patients, it is particularly common in individuals with acne rosacea, a chronic inflammatory skin condition. The good news is that IPL treatments can address the root causes of this form of MGD, providing patients with more lasting relief.
Another light-based therapy treatment—low-level light therapy (LLLT)—employs red and near-infrared light wavelengths, typically within the range of 590nm to 633nm and 740nm, allowing deeper penetration beneath the skin. It works by promoting biomodulation in cellular metabolism, which helps repair damaged cells and enhance normal cellular functions, along with providing analgesic and anti-inflammatory benefits.6 In contrast to IPL, LLLT is safe for all skin types and can be administered either as a standalone treatment or in combination with other therapies. Several studies have shown increased efficacy of the treatment results and significant improvements in signs and symptoms of MGD when combining IPL with LLLT. 7,8
For more severe cases of MGD, meibomian gland probing is a minimally invasive procedure that physically opens blocked gland ducts using 1mm, 2mm and 4mm probes. A common finding during the procedure is mechanical resistance when inserting the probe into the meibomian gland ducts. This resistance is thought to result from periductal fibrosis contracting around the orifice, leading to partial or complete obstruction of meibum flow. Probing relieves this resistance and restores gland function, improving meibum secretion and ocular surface health.9 Research supports combining gland probing with IPL or thermal treatments for even greater effectiveness in stabilizing the tear film and improving overall gland function.10,11
 |
Fig. 1. A patient undergoing manual meibomian gland expression (MGE) to re-establish normal secretory function. Click image to enlarge. |
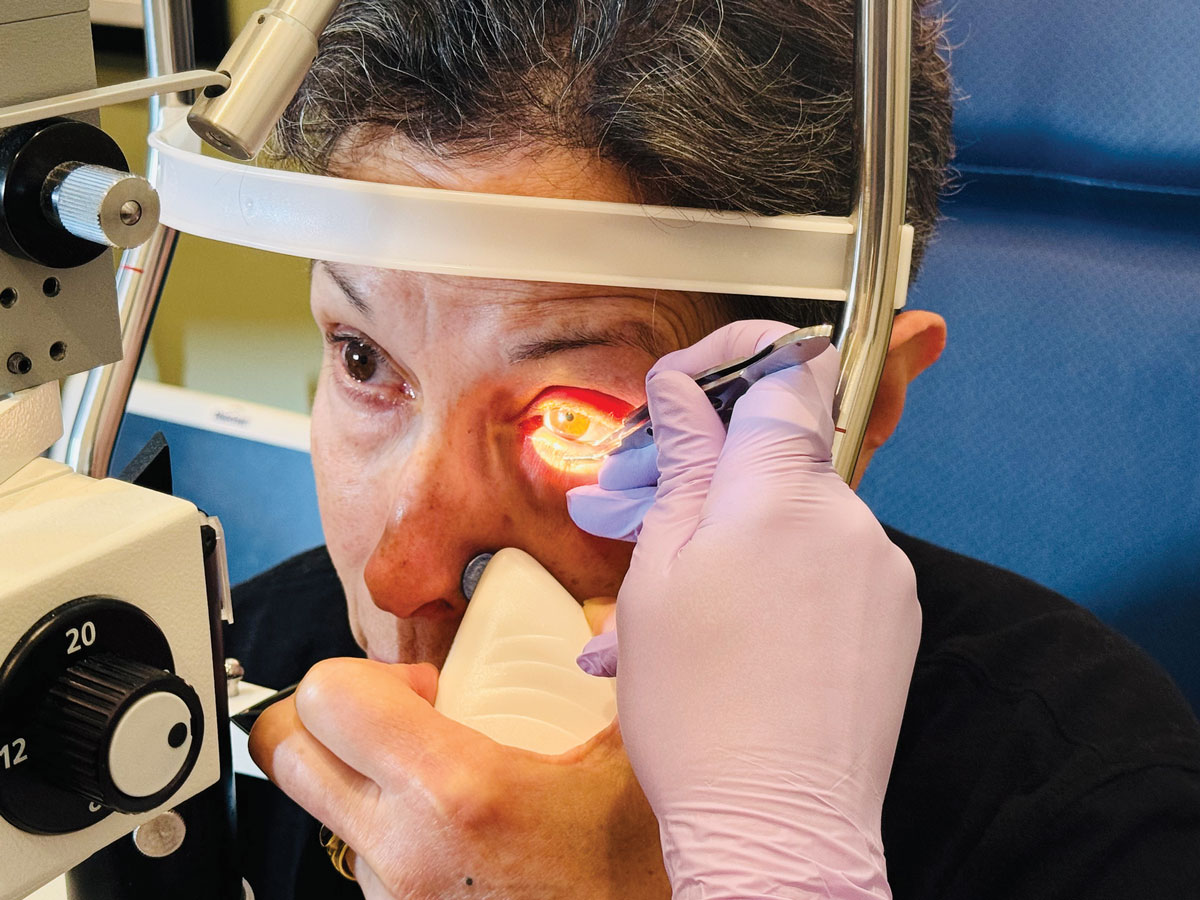 |
Fig. 2. iTear100 is an in-office treatment that stmulates the meibomian glands and improves meibum secretion during MGE. Click image to enlarge. |
Neurostimulation
A newer therapeutic strategy for managing DED that emerged a few years ago is neurostimulation of the nasolacrimal reflex. This reflex begins with the stimulation of the anterior ethmoidal nerve located in the nasal mucosa through chemical, mechanical or electrical means. The afferent pathway travels via the nasociliary branch of the ophthalmic division of the trigeminal nerve, reaching the midbrain, where it synapses in the superior salivatory nucleus of the pons.12 From there, the efferent pathway extends to the main and accessory lacrimal glands, as well as the meibomian glands and conjunctival goblet cells. This suggests that neurostimulation can effectively influence the three primary components of the tear film—mucin, aqueous and lipid—underscoring its potential in the comprehensive treatment of DED.
There are two neurostimulation options available at the moment, each with a distinct method of delivery.
Varenicline (Tyrvaya, Viatris Pharmaceuticals) is nasal spray for dry eye that acts as a cholinergic agonist. By stimulating nicotinic acetylcholine receptors in the nasal mucosa, Tyrvaya activates the trigeminal parasympathetic pathway, leading to increased tear production. A systematic review and meta-analysis showed that using Tyrvaya twice a day for 28 days significantly improves tear production, as measured by Schirmer scores.13 Patients need to be properly educated on use of the device to reduce undesirable side effects (e.g., excessive sneezing), which can deter routine use.
For those not interested in a nasal spray, a handheld device called iTear100 (Olympic Ophthalmics) also triggers the nasolacrimal reflex. About the size of a palm, this compact device contacts the skin at the junction of the nasal cartilage and nasal bone, where the external nasal nerve exits. When activated by the patient, the tip oscillates at a frequency of 220Hz to 270Hz and an amplitude of 0.5mm to 1mm, operating for up to 30 seconds on each side.12 Although designed as a prescription device, it can also be used in-office as an adjunct treatment to stimulate the meibomian glands, enhancing their expressibility and improving meibum secretion during MGE (Figure 2).
Pharmaceutical Options
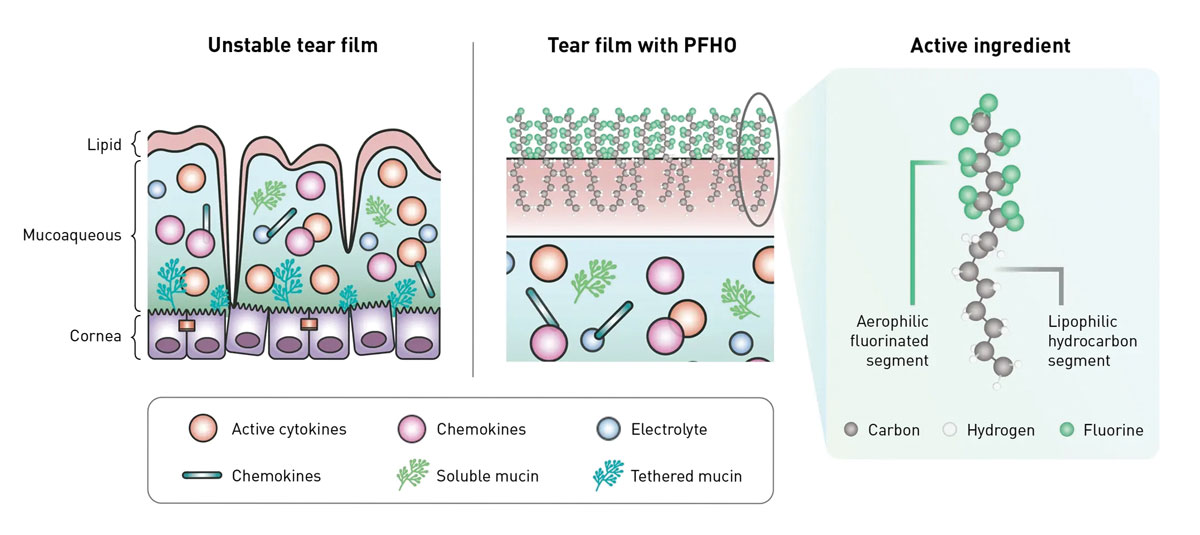
In 2023, Bausch + Lomb launched Miebo, the first prescription drug to directly address tear evaporation caused by MGD. Miebo is 100% perfluorohexyloctane with no vehicle, water, preservative or inactive ingredients. It is a physiologically inert substance with amphiphilic properties, i.e., the ability to attach to both air and lipid molecules (Figure 3). These properties enable Miebo to form a monolayer atop the tear film at the interface of the tears and the environment, spreading rapidly upon application to create a stable, anti-evaporative layer said to last up to six hours.14 This action helps stabilize the tear film, reduce friction and support ocular surface healing by mimicking natural meibum, which helps prevent excessive tear evaporation.
In clinical trials, Miebo showed significant improvements in eye dryness score and the total corneal fluorescein staining as early as day 15 (secondary endpoint) through day 57 (primary endpoint), with the most common side effects being mild blurred vision.15,16 These results make Miebo a promising new treatment for patients with MGD-related evaporative dry eye.
 |
|
Fig. 3. Perfluorohexyloctane, the active ingredient in Miebo, binds to both air and lipid molecules upon instillation. In this way, it compensates for tear instability due to inadequate lipid secretions by establishing its own barrier. Click image to enlarge. |
In clinical trials, Miebo showed significant improvements in eye dryness score and the total corneal fluorescein staining as early as day 15 (secondary endpoint) through day 57 (primary endpoint), with the most common side effects being mild blurred vision.15,16 These results make Miebo a promising new treatment for patients with MGD-related evaporative dry eye.
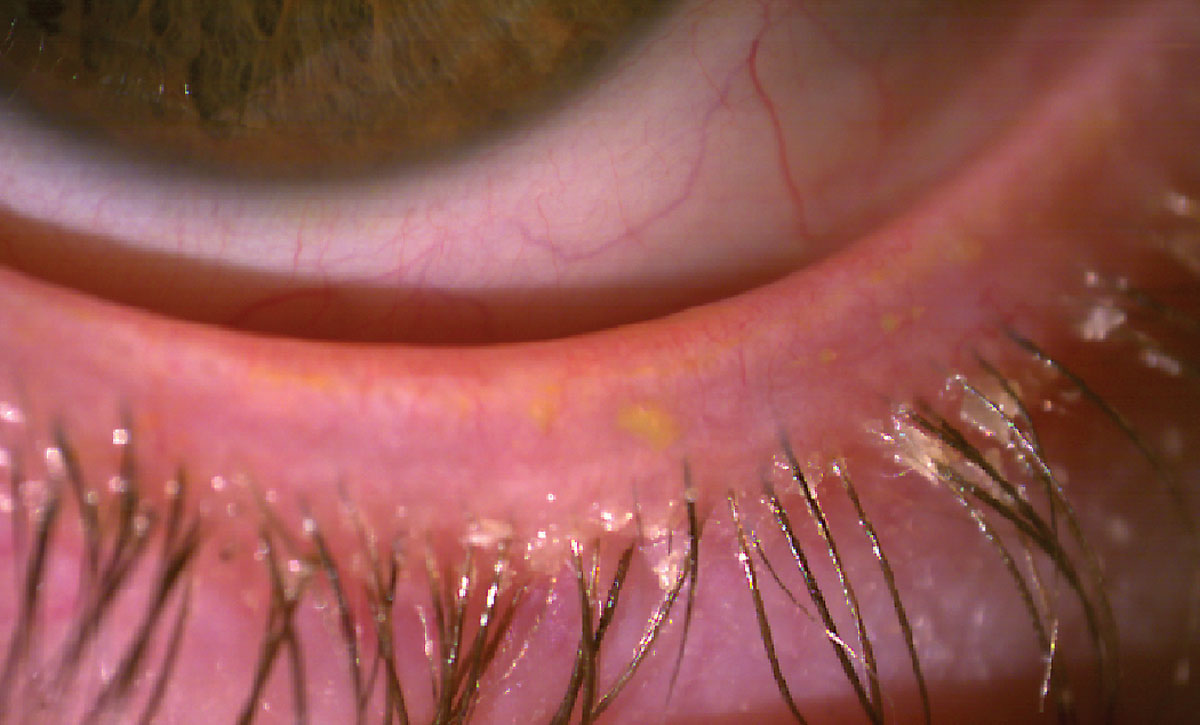
Lotilaner (Xdemvy), another new pharmaceutical launched in 2023, has shown promising results in treating Demodex blepharitis and MGD. In a Phase IIa trial, lotilaner demonstrated significant improvements in key indicators of MGD, including increased meibomian gland secretion scores and a higher number of glands secreting clear liquid.17 Both BID and TID dosing regimens were effective, with notable improvements observed by day 85 of treatment. Lotilaner also alleviated associated symptoms often linked to Demodex infestation like collarette formation and lid margin erythema (Figure 4). These findings suggest that lotilaner could be an impactful therapeutic option for MGD by addressing underlying causes rather than just managing symptoms.
Antibiotics like azithromycin and tetracyclines, though not new to MGD treatment, have gained increased attention for their anti-inflammatory effects.18,19 Recent studies indicate that topical azithromycin may be more effective than oral formulations in enhancing tear film stability and improving meibum quality.18 However, it carries a higher risk of local irritation.18
Tetracyclines, particularly doxycycline, remain as treatment options for reducing inflammation and supporting meibomian gland function. New dosing protocols have been introduced to minimize long-term side effects, improving the safety of prolonged use.
In a recent randomized clinical trial, researchers compared the efficacy of a three-week regimen of oral azithromycin (1g weekly) to a six-week course of oral doxycycline (200mg daily) for moderate to severe MGD. Both treatments were equally effective, significantly improving MGD and Ocular Surface Disease Index (OSDI) scores at six and eight weeks. The MGD score reductions were 5.31 for azithromycin and 4.97 for doxycycline at six weeks, with similar improvements by week eight. Importantly, azithromycin had fewer gastrointestinal side effects than doxycycline, making it a more tolerable option for some patients.19 These findings suggest that azithromycin could be an effective alternative to doxycycline for MGD treatment. However, further studies are needed to assess long-term outcomes and the potential adverse effects on the gastrointestinal system must be considered with these medications.
In the pipeline is AZR-MD-001 (Azura Ophthalmics), a promising selenium sulfide–based ophthalmic ointment currently advancing through Phase III clinical trials. In its Phase II trial, this ointment significantly improved gland function by opening an average of 4.2 glands and also reduced OSDI scores, therefore meeting primary endpoints at month three with a statistically significant improvement in the signs and symptoms of MGD.20 The ongoing trial aims to further confirm its efficacy and safety. If successful, AZR-MD-001 could offer another unique treatment specifically targeting MGD.
 |
|
Fig 4. Collarettes at the eyelash base typical of Demodex blepharitis. Click image to enlarge. |
Diet and Lifestyle Modifications
The 2023 Tear Film and Ocular Surface Society (TFOS) released another comprehensive assessment of dry eye, called the TFOS Lifestyle Report. It emphasized the significant impact of nutrition and lifestyle choices by individuals on DED, providing strong evidence linking metabolic diseases, particularly hyperlipidemia and diabetes, to the condition. It also reinforced the importance of dietary interventions in managing MGD.21
Dietary intake of omega fatty acids remain highly recommended for their anti-inflammatory properties, with studies suggesting that a lower omega-6 to omega-3 ratio leads to improved MGD outcomes.21 Omega-3 and omega-6 polyunsaturated fatty acids are among the most studied dietary supplements for OSD, though some findings are contradictory. However, based on generally favorable safety profile, omega-3 fatty acids and omega-6 gamma-linolenic acid may still offer a valuable treatment option for patients with DED and MGD.
Deficiencies in vitamins A, B12, C and D have all been linked to dry eye. Additionally, there is growing evidence of the role vitamin D plays in MGD management.21 Research shows that higher vitamin D levels are associated with better meibomian gland function and reduced inflammation, likely due to its role in immune response regulation and lipid metabolism.21,22
Of note, one mouse study investigated the effects of a high-fat diet on MGD. A high-fat diet led to lipid accumulation in meibomian glands, increased inflammation and upregulation of inflammatory markers such as IL-1β, TNF-α and IL-6.23 The diet also reduced a key regulator of lipid metabolism and inflammation known as PPAR-γ. This resulted in cell apoptosis and gland dysfunction. However, switching the mice back to a standard diet and treating them with an PPAR-γ agonist reversed many of the inflammatory and dysfunctional effects. The study highlights the potential role of diet and PPAR-γ modulation in treating hyperlipidemia-induced MGD.
Sleep is another important lifestyle factor influencing both MGD and DED, as evidenced by a study that found a significant link between dry eye and poor sleep quality.24 Another study comprehensively examined the relationship between sleep quality and DED, focusing on meibomian gland dropout. It showed that individuals with poor sleep quality show more severe glandular dropout, lower tear break-up time and higher meibum grade scores compared to those with better sleep.25 These results align with previous studies linking sleep disorders to DED severity and underscore the importance of evaluating sleep quality in dry eye patients and supports the need for integrated treatment approaches that consider comorbid conditions like depression, anxiety and systemic diseases.
Additionally, obstructive sleep apnea-hypopnea syndrome has been shown to exacerbate MGD. The chronic intermittent hypoxia associated with this sleep disorder increases oxidative stress and inflammation, damaging the meibomian glands, leading to gland dropout, plugged orifices and altered meibum secretion.26
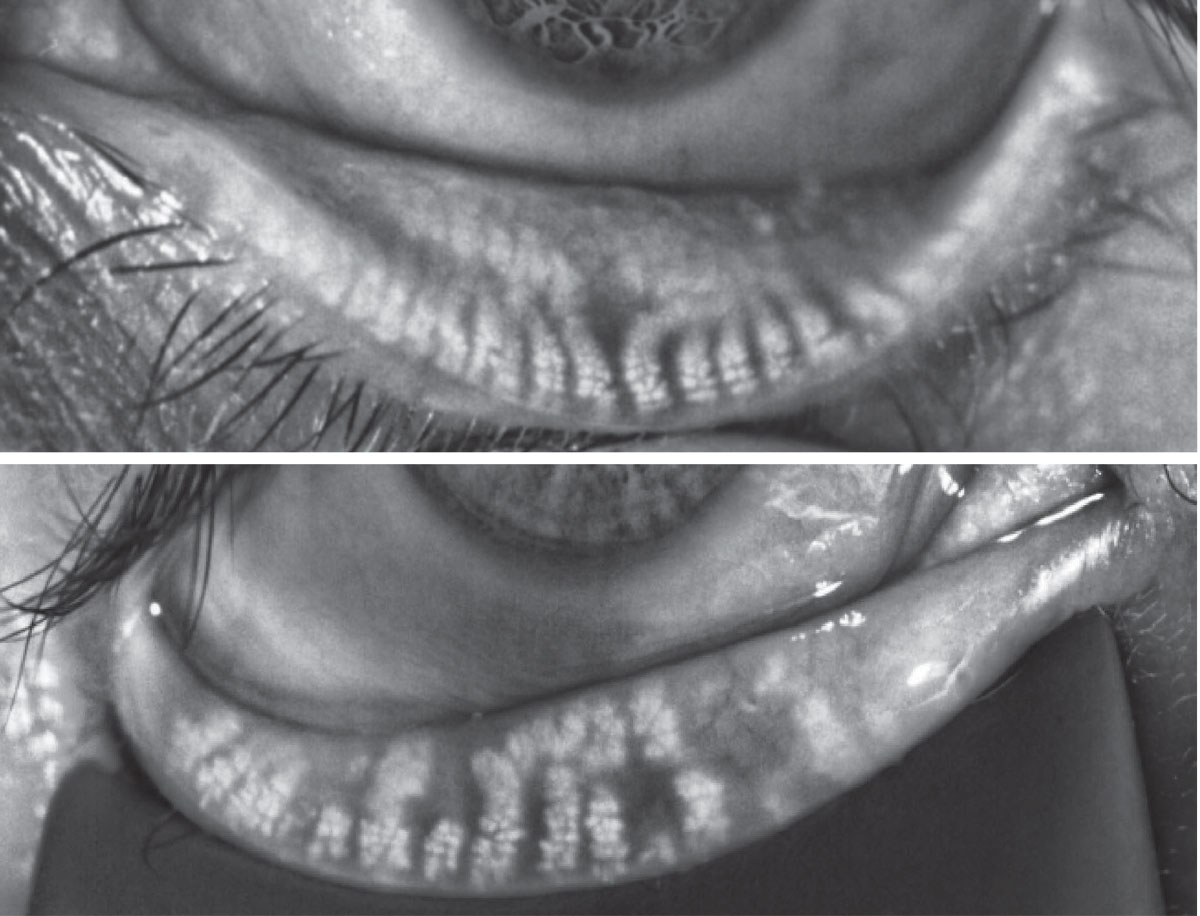 |
Fig. 4. Miebography images showing normal gland anatomy (top) vs. a presentation characterized by gland dilation and atrophy (bottom). Click image to enlarge. |
Takeaways
The ever-evolving treatment landscape for MGD offers more hope for patients with chronic evaporative DED. Whether through in-office procedures like IPL, novel eye drops such as Meibo or lifestyle modifications involving omega-fatty acids and vitamin D, the emphasis is increasingly on therapies that not only provide symptomatic relief but restore meibomian gland function. As research continues, individualized treatment plans that combine multiple modalities will become the gold standard for MGD management, ensuring better symptom control and an improved quality of life for patients.
Dr. Ioussifova graduated from the New England College of Optometry and completed a residency program in community health and ocular disease in Boston. She is a fellow of the American Academy of Optometry, previously served as an adjunct clinical faculty at the Pacific University College of Optometry and was an examiner for the NBEO. Dr. Ioussifova owns South Waterfront Eye Center, a practice with special interests in advanced dry eye treatments, nutritional counseling and aesthetic services. She has no financial disclosures.
1. Chelnis J, Garcia CN, Hamza H. Multi-frequency RF combined with intense pulsed light improves signs and symptoms of dry eye disease due to meibomian gland dysfunction. Clin Ophthalmol. 2023;17;3089-102. 2. Tashbayev B, Yazdani M, Arita R, et al. Intense pulsed light treatment in meibomian gland dysfunction: a concise review. Ocul Surf. 2020;18(4):583-94. 3. Pac CP, Munteanu M, Sánchez-González JM, et al. Long-term impacts of intense pulsed light therapy on ocular surface health and tear film dynamics in patients with dry eye disease: detailed analysis and observations over a one-year follow-up period. Opthalmol Ther. 2024;13(10):2715-30. 4. Sheppard JD, Nichols KK. Dry eye disease associated with meibomian gland dysfunction: focus on tear film characteristics and the therapeutic landscape. Ophthalmol Ther. 2023;12(3):1397-418. 5. Peng K-L, Chiu C-J, Tuan H-I, et al. Combination treatment of intense pulsed light therapy and meibomian gland expression for evaporative dry eye. Life (Basel). 2022;12(7):1086. 6. Park Y, Kim H, Kim S, Cho KJ. Effect of low-level light therapy in patients with dry eye: a prospective, randomized, observer-masked trial. Sci Rep. 2019;12(1):3575. 7. D’Souza S, James E, Koul A, et al. A randomized controlled study evaluating outcomes of intense pulsed light and low-level light therapy for treating meibomian gland dysfunction and evaporative dry eye. Indian J Opthalmol. 2023;71(4):1608-12. 8. Ballesteros-Sánchez A, Gargallo-Martínez B, Sánchez-González MC, Sánchez-González, JM. Intense pulsed light and low-level light therapy for treating meibomian gland dysfunction and evaporative dry eye. Indian J Opthalmol. 2023;71(12):3730. 9. Maskin SL, Alluri S. Intraductal meibomian gland probing: background, patient selection, procedure and perspectives. Clin Ophthamol. 2019;13:1203-23. 10. Magno M, Moschowits E, Arita R, et al. Intraductal meibomian gland probing and its efficacy in the treatment of meibomian gland dysfunction. Surv Ophthalmol. 2021;66(4):612-22. 11. Huang X, Qin Q, Wang L, et al. Clinical results of intraductal meibomian gland probing combined with intense pulsed light in treating patients with refractory obstructive meibomian gland dysfunction: a randomized controlled trial. BMC Ophthalmol. 2019;19(1):211. 12. Yu MD, Park JK, Kossler AL. Stimulating tear production: spotlight on neurostimulation. Clin Ophthalmol. 2021;15:4219-26. 13. Frampton JE. Varenicline solution nasal spray: a review in dry eye disease. Drugs. 2022;82(14):1481-8. 14. Sheppard JD, Evans DG, Protzko EE. A review of the first anti-evaporative prescription treatment for dry eye disease: perfluorohexyloctane ophthalmic solution. Am J Manag Care. 2023;29(14 Suppl):S251-9. 15. Sheppard JD, Kurata F, Epitropoulos AT, et al. NOV03 for signs and symptoms of dry eye disease associated with meibomian gland dysfunction: the randomized phase 3 MOJAVE Study. Am J Opthalmol. 2023;252:265-74. 16. Craig JP, Alves M, Wolffsohn JS, et al. TFOS Lifestyle Report Executive Summary: a lifestyle epidemic - ocular surface disease. Ocul Surf. 2023;30:240-53. 17. Syed YY. Lotilaner ophthalmic solution 0.25%: first approval. Drugs. 2023;83(16):1537-41. 18. Tao T, Tao L. Systematic review and meta-analysis of treating meibomian gland dysfunction with azithromycin. Eye (Lond). 2020;34(10):1797-808. 19. Upaphong P, Tangmonkongvoragul C, Phinyo P. Pulsed oral azithromycin vs six-week oral doxycycline for moderate to severe meibomian gland dysfunction: a randomized clinical trial. JAMA Ophthalmol. 2023;141(5):423-9. 20. Watson SL, Jones LW, Stapleton F, et al. Efficacy and safety of AZR-MD-001 selenium sulfide ophthalmic ointment in adults with meibomian gland dysfunction: a vehicle-controlled, randomized clinical trial. Ocul Surf. 2023;29:537-46. 21. Markoulli M, Ahmad S, Arcot J, et al. TFOS Lifestyle: Impact of nutrition on the ocular surface. Ocul Surf. 2023;29:226-71. 22. Fukuoka S, Arita R, Mizoguchi T, et al. Relation of dietary fatty acids and vitamin D to the prevalence of meibomian gland dysfunction in Japanese adults: the Hirado-Takushima Study. J Clin Med. 2021;10(2):350. 23. Bu J, Zhang M, Wu Y, et al. High-fat diet induces inflammation of meibomian gland. Invest Ophthalmol Vis Sci. 2021;62(10):13. 24. Magno MS, Utheim TP, Snieder H, et al. The relationship between dry eye and sleep quality. Ocul Surf. 2021;20:13-9. 25. Zhu Y, Huang X, Lin L, et al. Sleep quality is associated with severe meibomian gland disruption in dry eye. Front Med (Lausanne). 2022;9:812705. 26. Liu S, Li S, Li M, et al. Evaluation of the ocular surface and meibomian gland in obstructive sleep apnea hypopnea syndrome. Front Med (Lausanne). 2022;9:832954. |

