2023 Pharma IssueIn the March issue of Review of Optometry, experts provide exciting updates on incoming dry eye treatments, oral steroid prescribing protocols and advancements in presbyopia management. Plus, learn how to fight insurance denials for new meds. Check out the other articles featured in this issue:
|
Dry eye disease (DED) affects all pillars of our practices from simple refractions to systemic disease comanagement, and it of course dramatically affects our patients, disrupting their daily lives. Newer diagnostics and treatments, and the advanced technology and research behind them, are helping us better care for our patients, though.
Many new medications are making their way through the FDA approval process, and a few were added to our armamentarium over the last few years. By the end of 2023, we’re hopeful to have two more drugs to add to the DED toolbox.
Let’s dive into the newer drugs, how they work and where we fit them into our current clinic protocols.
Tyrvaya (varenicline tartrate solution 0.03mg, Viatris)
Approved by the FDA in October 2021, Tyrvaya is the first nasal spray to treat the signs and symptoms of DED. It is applied to the inside of the lower nasal area twice a day.
Mechanism of action: Although the exact mechanism is still unknown, it is believed to cause cholinergic neuro-activation via the trigeminal parasympathetic pathway, which is understood to play a role in tear film stability by stimulating the lacrimal functional unit.1,2 Stimulation to the lacrimal functional unit increases basal tear film production, which makes up about 34% of the tear film and helps to relieve dry eyes.3 Basal tears contain a complex mix of 2,000 molecules. Imbalance of the tear film leads to loss of tear film homeostasis, resulting in dessication and an inflammatory cascade that can cause DED.4 Restoring the tear film was shown in clinical trials to improve both symptoms, using the Eye Dryness Score (EDS), and signs measured by Schirmer testing.5
Side effects: Tyrvaya is not reported to cause any significant adverse events to the ocular surface; however, it does have non-ocular side effects, including sneezing (82%), coughing (16%), throat irritation (13%) and instillation site irritation (8%).5,6,7
Why choose Tyrvaya: As a nasal spray, it’s the first option that can spare the ocular surface from drug exposure. A review conducted in 2022 on the use of Tyrvaya reported that this agent is well-tolerated and advantageous over the available topical medications.8
In clinical trials, study participants increased their Schirmer score after using Tyrvaya BID for four weeks.5,6 In a prospective study where participants used cyclosporine 0.005% ophthalmic solution BID for six months, 35.1% achieved ≥5mm improvement and 18.9% achieved ≥10mm improvement in the average Schirmer scores.9
 |
|
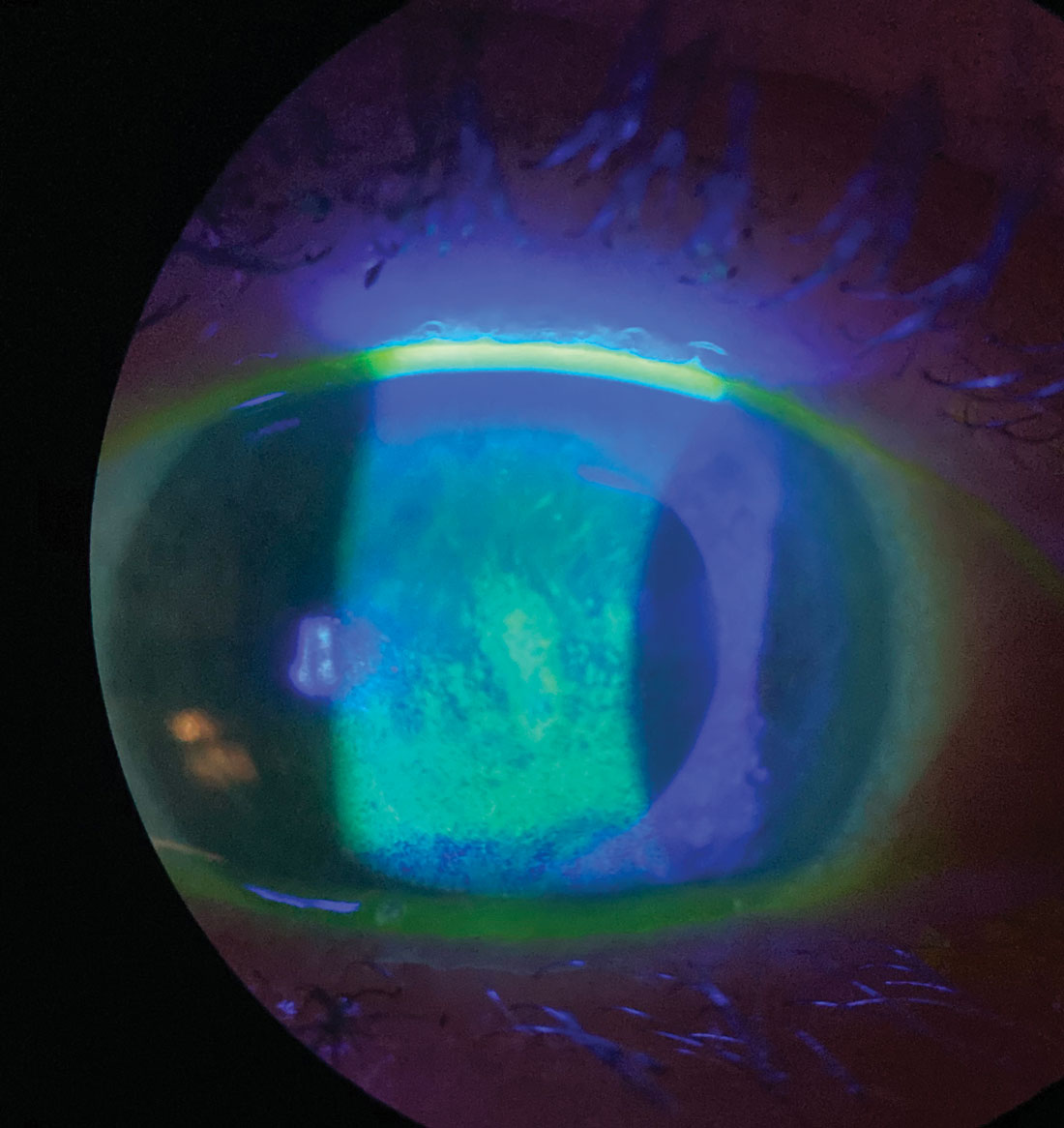
Sodium fluorescein staining shows this patient’s corneal surface is compromised by dry eye disease. Click image to enlarge. |
Cequa (cyclosporine 0.09%, Sun Ophthalmics)
Approved by the FDA in 2018, Cequa offers a 0.09% concentration formulation of the familiar drug cyclosporine in an aqueous nanomicellar formulation. It increases tear production in DED patients, and its use of nanomicelles—small, highly soluble colloids with a hydrophilic surface—for drug delivery aims to increase cyclosporine bioavailability while reducing adverse reactions.10
Mechanism of action: Cequa is believed to work as an immunomodulator. Cyclosporine increases tear production in patients where it’s presumed to be suppressed due to ocular inflammation associated with DED.11 It inhibits the activation of transcription factors required for T-cell activation and inflammatory cytokine production, which decreases inflammation in the eye, leading to an increase in tear production.12
Cequa is unique due to its nanomicellar delivery system. The micelle formation has a hydrophobic core and an outer water-soluble or hydrophilic shell. These nanomicelles are thus more bioavailable in the precorneal tear film.9
Side effects: Ocular adverse events were mild and transient irritation, and no serious ocular adverse effects were reported in the Phase II and III studies.11
Why choose Cequa: The higher concentration and improved drug delivery method may be more effective and better tolerated than older forms of cyclosporine.14,15
After 14 days of BID dosing, cyclosporine 0.09% demonstrated a numerically greater treatment effect in conjunctival staining, corneal staining and tear breakup time (TBUT), and at six months, after taking cyclosporine 0.005%, TBUT improved by more than 50% in both eyes.9
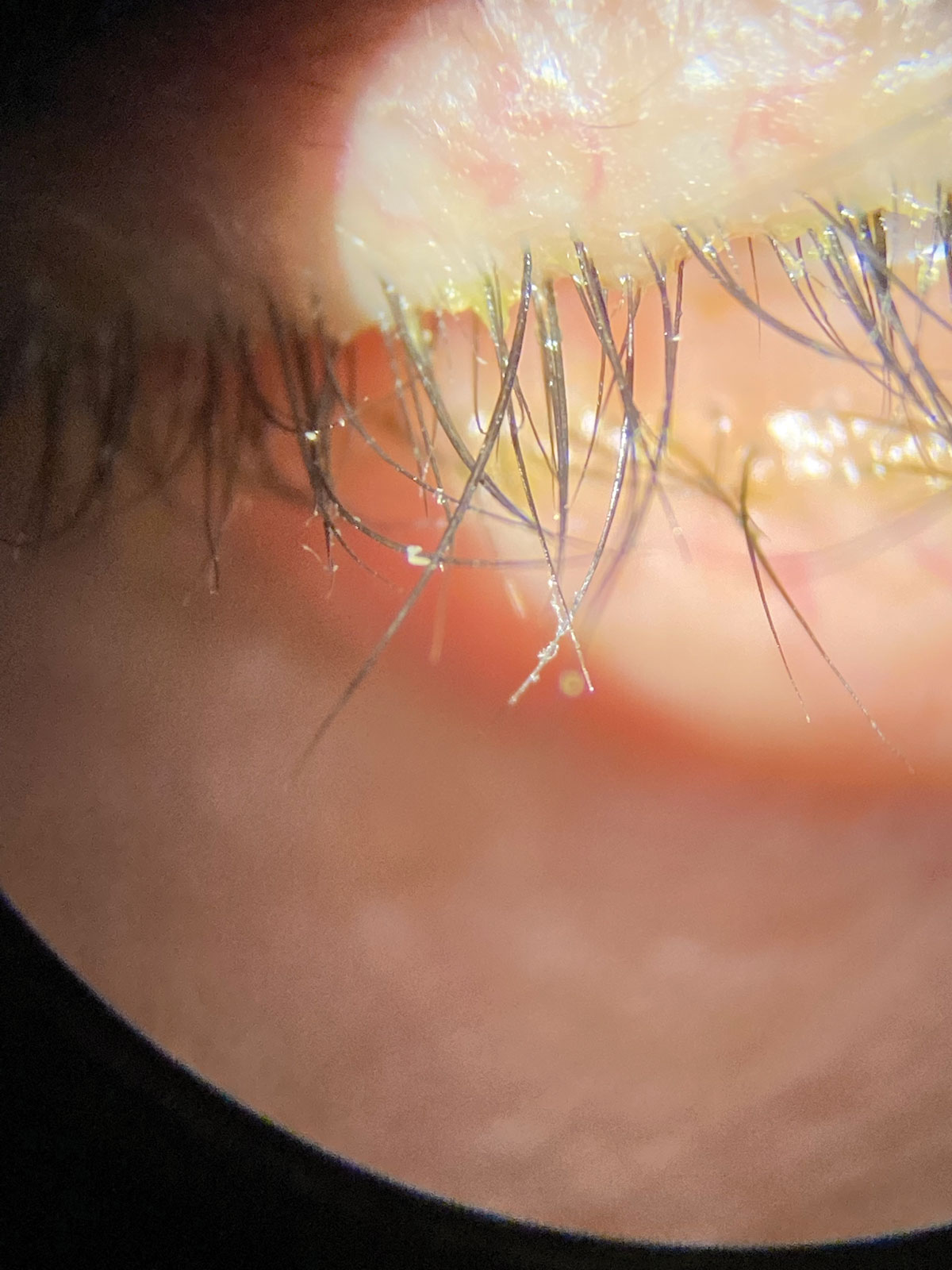 |
Collarettes are pathognomonic for Demodex blepharitis. Click image to enlarge. |
Eysuvis (loteprednol etabonate ophthalmic suspension 0.25%, Alcon)
This corticosteroid eye drop is used for the treatment of DED flares. In 2020, it became the first topical steroid to be approved by the FDA for the short-term treatment of dry eye. In one of its clinical trials, Eysuvis was found to be significantly effective for treatment of hyperemia and dry eye without elevating intraocular pressure (IOP).16
Mechanism of action: Corticosteroids work by suppressing cellular infiltration, capillary dilation, fibroblast proliferation and collagen deposition.17 They also interfere with the synthesis of pro-inflammatory molecules and they inhibit scar formation.18
Eysuvis is an ophthalmic nanosuspension that delivers corticosteroid to the anterior eye using mucus-penetrating particles (MPPs). This delivery concept facilitates the drug penetration through the mucus barrier and enables the drug to spread more quickly and uniformly across the ocular surface than in conventional processes.19 These are favorable properties for treatment of ocular surface disease.16
Side effects: Although corticosteroids are an effective treatment for DED, long-term use is associated with cataract development, increased IOP and opportunistic infections.20 Eysuvis breaks down rapidly after administration to the ocular surface and reduces the risk of elevated IOP and cataract formation associated with other topical steroids.16
Why choose Eysuvis: In pre-clinical studies, 0.5% loteprednol vs. 0.4% loteprednol in MPP formulation was shown to have a 3.6-fold higher concentration in the corneal epithelium after just five minutes.21 Eysuvis is used for short periods, so the side effects are minimal compared to traditional corticosteroids.16 Eysuvis is the first FDA-approved ocular steroid indicated for DED, though off-label use of others, notably Lotemax (Bausch + Lomb), has long been recognized as effective.20
 |
|
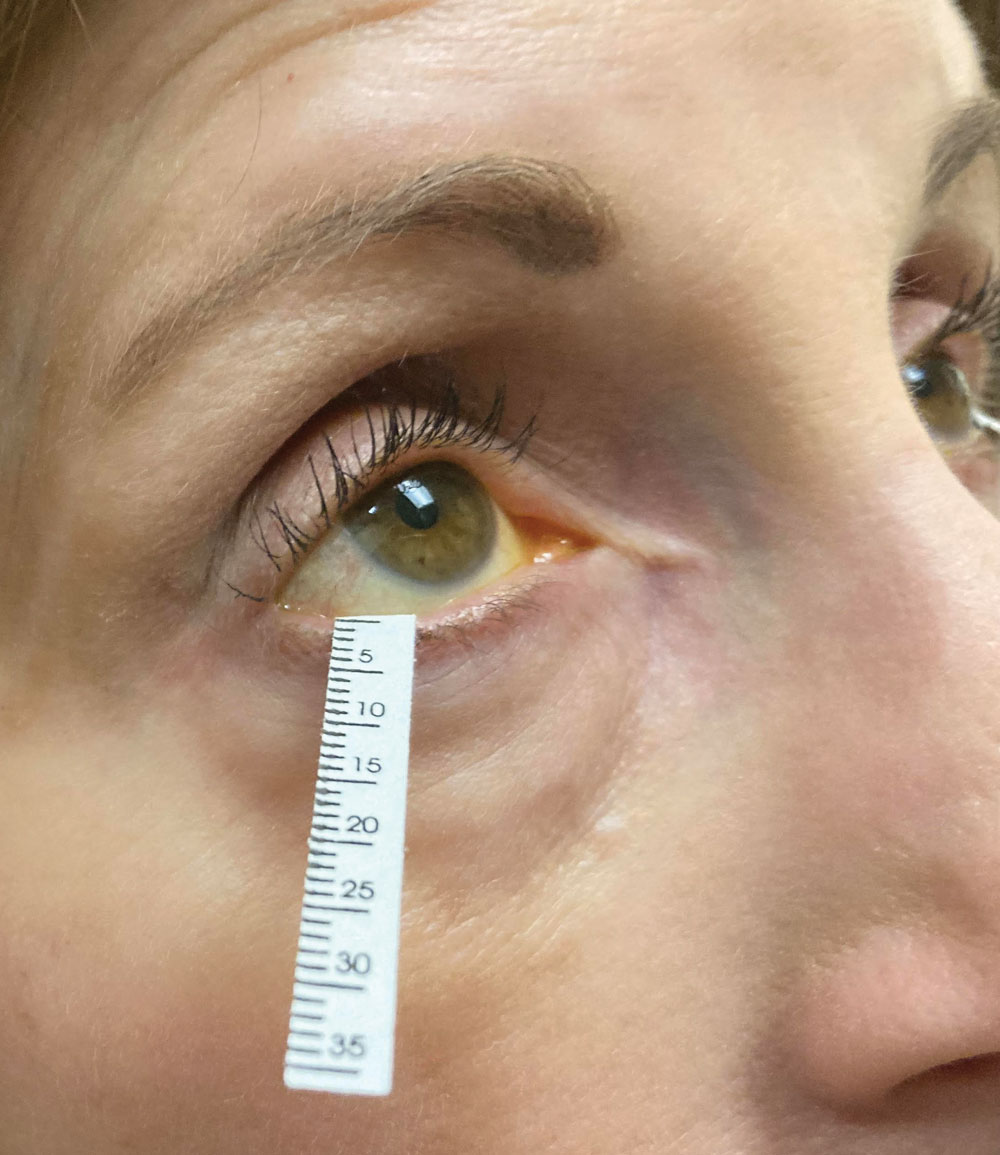
Schirmer’s strip in use. Its scores are a mainstay of clinical trials of drug performance as well as clinical assessment by ODs. Photo: Jessica Steen, OD. Click image to enlarge. |
Regener-Eyes (Regener-Eyes)
This is a sterile, preservative-free option to relieve dryness of the eye that is nonsteroidal and derived from amniotic fluid.23 According to the company, use of Regener-Eyes assists the tear film layer by increasing lubrication and hydration, which promotes homeostasis of the corneal surface and helps slow down the cycle of dry eye perpetuation. It is well-tolerated in patients, with the most common side effect being instillation site irritation. This drop does not have an FDA approved label and as such is an over-the-counter product.
 |
Inveltys (loteprednol etabonate ophthalmic suspension 1%, Alcon)
This drug was FDA-approved in August 2018 and is the only twice-daily ocular corticosteroid indicated for the treatment of inflammation and pain after ocular surgery.39 It came to the market to meet the needs of surgeons who were looking for a drop that could control postoperative inflammation without elevating IOP.39 Naturally, off-label use is widespread and that includes for appropriate dry eye patients.
Mechanism of action: As is the case with Eysuvis, the reliable efficacy of loteprednol etabonate gets a boost from MPP. According to the company, this allows more of the drug to penetrate through the mucins directly to the target ocular tissue. One study supports the premise that the MPP use can enhance ocular exposure of topically applied therapeutic agents.21
The mucins in the tear film have a protective effect, eliminating pathogens and other insults. In doing so, they also may limit the penetration of traditional suspension eye drops, as they can be rapidly cleared.41
Why choose Inveltys: Loteprednol consistently demonstrates a low propensity to elevate IOP, regardless of formulation, dosage regimen or treatment duration, including in known steroid responders.27
 |
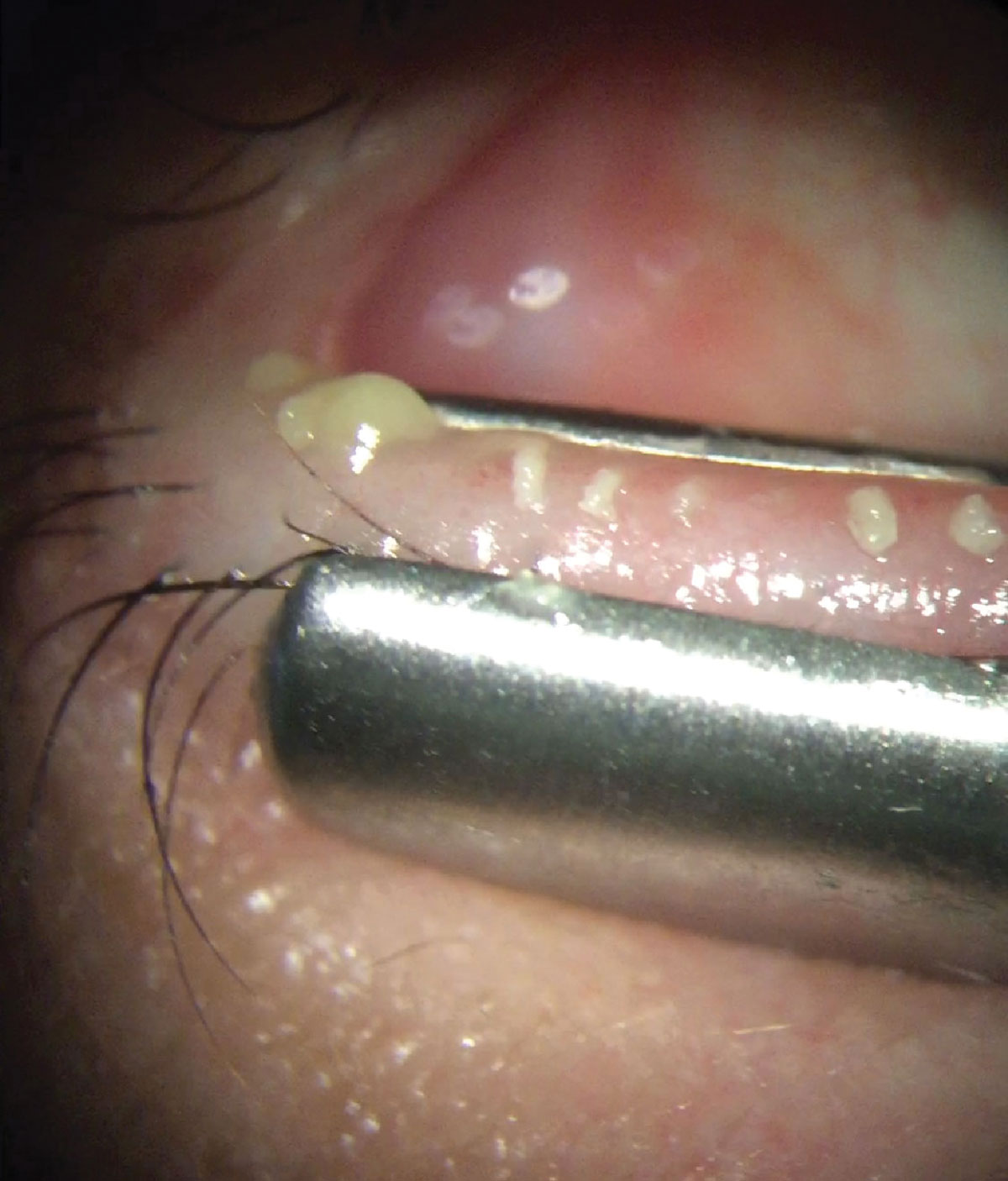
Reproxalap (Aldeyra Therapeutics)
This agent is a reactive aldehyde species (RASP) inhibitor. RASP is elevated in ocular (allergic conjunctivitis and DED) and systemic inflammatory diseases. In the eyes, RASP can cause many symptoms including inflammation, redness, change lipid tear composition and decreased tear production.
Mechanism of action: Reproxalap may represent a new therapeutic approach for the treatment of DED. It works as a nonsteroidal anti-inflammatory by binding to RASP. In dry eye patients, reproxalap dosed QID results in reduction of inflammation and stabilization of the tear film by preventing RASP modification of tear lipids.
Clinical trials: Reproxalap 0.25% dosed QID over 12 weeks demonstrated rapid, broad and clinically relevant symptomatic control of DED as measured by OSDI and SANDE, and DED signs improved as demonstrated by fluorescein staining.
Adverse events: Although reproxalap is an anti-inflammatory, it has not been shown to cause elevated IOP in clinical trials. The drug has been well tolerated and symptoms have been reduced with QID dosing in as little as one week.
The NDA PDUFA date for reproxalap has been projected as June 8, 2023.
 |
|
To minimize ocular surface drug exposure while also providing complementary therapy, many practices offer in-office procedures such as meibomian gland expression. Click image to enlarge. |
CyclASol (Novaliq)
This topical anti-inflammatory and immunomodulating ophthalmic drug solution contains 0.1% cyclosporine A in a unique water-free solution that the company calls EyeSol; it is believed to increase the tolerability of cyclosporine A on the eye, according to the company.
Mechanism of action: CyclASol’s water-free formulation enables the cyclosporine to have a longer duration on the ocular surface. EyeSol is said to provide a small drop size of a nonaqueous and clear solution. The drug is designed for improved ocular tolerability and local bioavailability, which should allow for earlier onset of efficacy compared to other formulations of cyclosporine A.28 The company says that CyclASol’s clear solution quickly spreads across the ocular surface due to its low surface tension. This minimizes the usual decrease in visual acuity that is seen in lipid-based eye drops, ointments and emulsions.28
Clinical trials: CyclASol was studied in a 12-week, double-blind multicentered clinical trial that compared it to vehicle at a BID dosing regimen. In the Essence trial, statistically significant improvements in central corneal staining were reached after two weeks of therapy. Also, statistically significant improvement in EDS was met after four weeks of use. CyclASol was found to have an earlier onset of action compared to other cyclosporine formulations.
Essence-2 study showed that CyclASol was able to maintain its reduction in the signs and symptoms of DED over a 52-week period.
The drug also demonstrated favorable tolerability. Essence-2 showed visual disturbances of 7.8%, similar to that of Restasis. The other adverse events reported (present in <2% of subjects) were blurred vision, instillation site pain and conjunctivitis.
The FDA has assigned a PDUFA date of June 8, 2023.
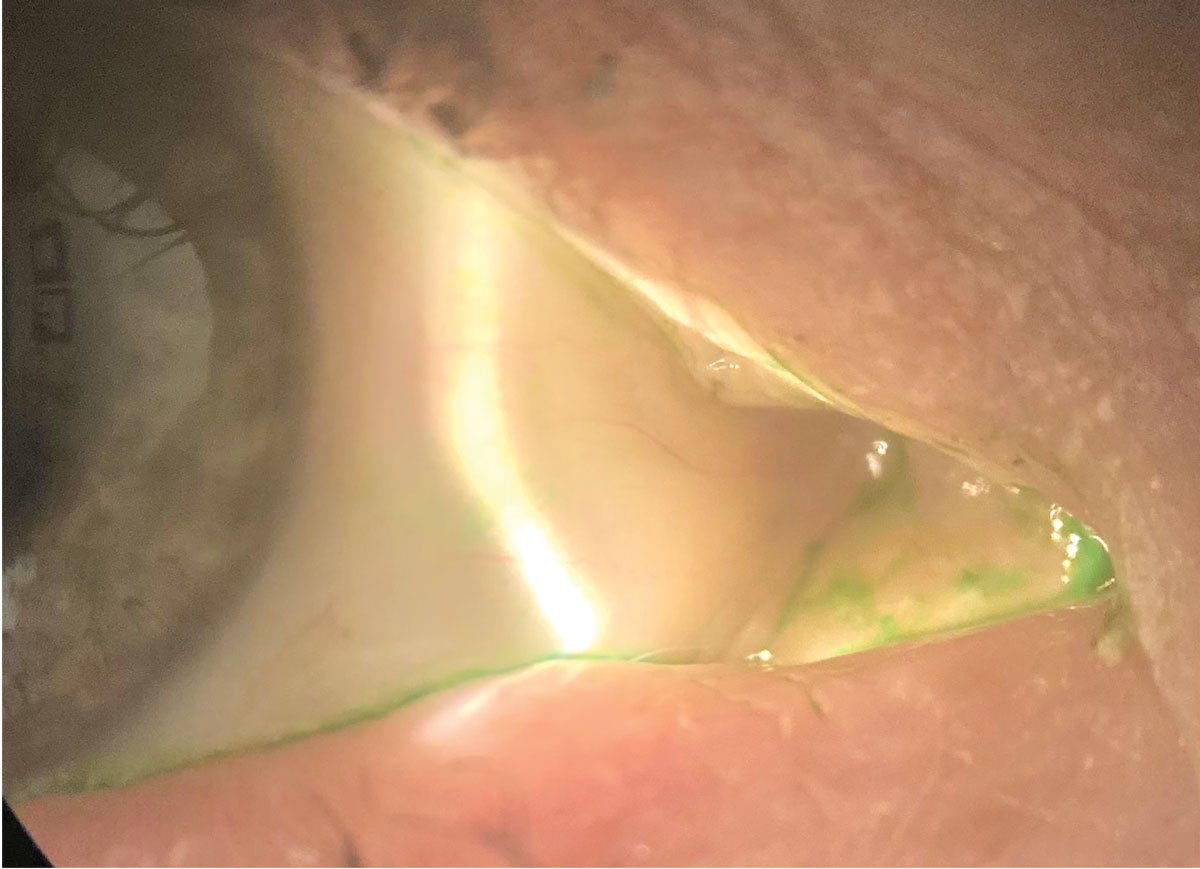 |
Here, the conjunctiva is stained with lissamine green. Cequa showed a reduction in conjunctival staining in its clinical trials. Click image to enlarge. |
NOV03 (Bausch + Lomb)
This investigational therapy is a preservative-free, water-free, nonsteroidal drop intended for treatment of DED with associated MGD.29
The active ingredient is perfluorohexyloctane. The product also contains the EyeSol formulation developed by Novaliq.
Mechanism of action: Its developers say that EyeSol allows the drop to stay on the eye up to 240 minutes, as opposed to traditional water-based topical agents, which last on the eye for three to five minutes. EyeSol consequently allows NOV03 to be up to four times more bioavailable than water-based drops. The use of EyeSol also allows NOV03 to penetrate the meibomian glands, the company says.
A Phase II trial successfully evaluated the efficacy, safety and tolerability of NOV03 in patients with DED associated with MGD. There was improvement in signs (corneal staining) and symptoms (by EDS).30 NOV03 spreads rapidly across the ocular surface because of its low surface tension.31 It does not cause blurred vision as ophthalmic gels or ointments do because its refractive index is similar to water.32
Both the signs and symptoms were statistically significantly reduced with QID dosing at two weeks.
Adverse events: The drop is said to be very comfortable and the only adverse event that occurred in more than 1% of study subjects was mild blepharitis. Even though the agent remains on the eye for several hours, patients did not report blurred vision.
NOV03 may offer an appealing new approach to care for the 86% of dry eye sufferers with some form of evaporative DED.33 Altered tear film lipid layer in MGD leads to tear film instability and evaporative aqueous loss. Treatments thus far have relied on interventional approaches treating the eyelids. Having a pharmaceutical adjust would be welcome by many practitioners.
 |
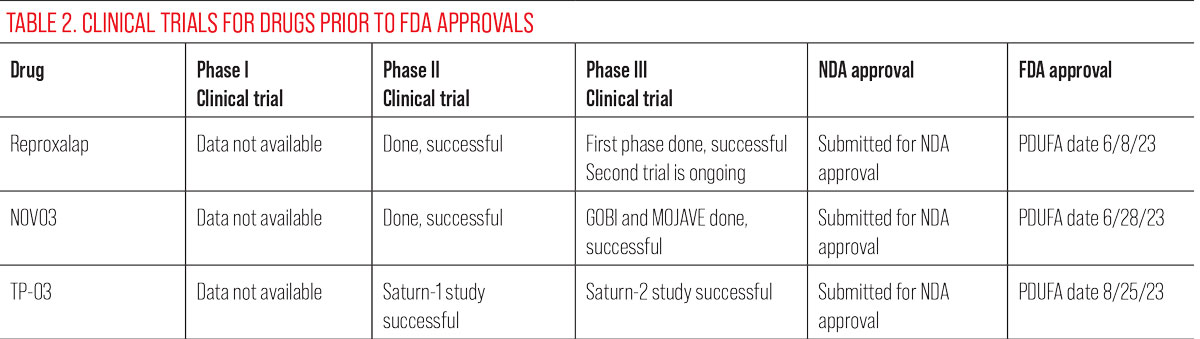
TP-03 (lotilaner ophthalmic solution, Tarsus Pharmaceuticals)
This drug is currently undergoing its NDA phase of FDA approvals. It is a topical ophthalmic eye drop intended for use twice daily for six weeks to help paralyze, eradicate and exterminate Demodex mites.35
Although Demodex blepharitis is not a form of DED per se, long-term Demodex infestations will clog the meibomian glands, decrease meibum production and lead to or exacerbate MGD.36,37 In such patients, eliminating Demodex could improve MGD, a root cause of evaporative DED.
Mechanism of action: Lotilaner, the active ingredient, uses parasite-specific GABA inhibition to paralyze the mites’ nervous system, which leads to death. Clinical trials show more than 80% of participants reached a clinically meaningful cure to their Demodex blepharitis.35
The PDUFA target date for TP-03 is August 25, 2023.
Takeaways
It’s an exciting time in our profession when contemplating treatment of our old foe dry eye, as many noteworthy new meds will certainly make a difference for you and your patients now and in the near future. Always remember to recommend preventive ulcer medications and calcium supplements for long-term steroid use. Understanding the mechanism of action of oral steroids, the most common uses in eye care and the side effects/contraindications to oral steroids makes prescribing them when indicated much less intimidating.
Dr. Theriot practices at a multi-specialty eye clinic in Shreveport, LA. Her clinical practice covers a broad spectrum of ocular care with a unique focus on ocular surface disease and dry eye. She received her doctorate in optometry from UC Berkeley School of Optometry and completed her residency at SUNY College of Optometry. She is a consultant for Novartis and key opinion leader for Sun Pharmaceuticals and Kala Pharmaceuticals.
Dr. Hornick practices at Stanford Ranch Optometry in Rocklin, CA. She received her doctorate in optometry from the Southern California College of Optometry. She is a Fellow of the American Academy of Optometry and a board member for the Sacramento Valley Optometric Association. She has no financial disclosures.
1. Dieckmann G, Fregni F, Hamrah P. Neurostimulation in dry eye disease - past, present and future. Ocul Surf. 2019;17(1):20-7. 2. Labetoulle M, Baudouin C, Calonge M, et al. A rrole of corneal nerves in ocular surface homeostasis and disease, Octa Ophthalmol. 2019;97(2):137-45. 3. Krader CG. Varenicline nasal spray approved as a treatment for dry eye disease. Ophthalmology Times. 2021;46(19). 4. Lam SM, Tong L, Duan X, et al. Extensive characterization of human tear fluid collected using different techniques unravels the presence of novel lipid amphiphiles. J Lipid Res. 2014;55(2):289-98. 5. Oyster Point Pharma. Data on file. OPP-002 (Onset-1) Clinical Study Report. August 19, 2021. Accessed February 20, 2023. 6. Oyster Point Pharma. OPP-101 (Onset-2) Interim Clinical Study Report. October 13, 2020. Accessed February 20, 2023. 7. Oyster Point Pharma. Data on file. OPP-004 (MYSTIC) Clinical Study Report. March 19, 2020. Accessed February 20, 2023. 8. Frampton JE. Varenicline solution nasal spray: a review in dry eye disease. Drugs. 2022;82(14):1481-8. 9. Stonecipher KG, Torkildsen GL, Ousler GW III, et al. The IMPACT study: a prospective evaluation of the effects of cyclosporine ophthalmic emulsion 0.05% on ocular surface staining and visual performance in patients with dry eye. Clin Ophthalmol. 2016;10:887-95. 10. Ames P, Galor A. Cyclosporine ophthalmic emulsions for the treatment of dry eye: a review of the clinical evidence. Clin Investig (Lond). 2015;5(3):267-85. 11. Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15(3):575-628. 12. de Oliveira RC, Wilson SE. Practical guidance for the use of cyclosporine ophthalmic solutions in the management of dry eye disease. Clin Ophthalmol. 2019;13:1115-22. 13. Hessen M. Cyclosporine Shoot-Out: how do they match up? Rev Optom. May 15, 2019. Accessed February 20, 2023. 14. Luchs J. Phase 3 clinical results of cyclosporine 0.09% in a new nanomicellar ophthalmic solution to treatment keratoconjunctivitis sicca. In: 2018 ASCRS ASOA Annual Meeting. ASCRS; 2018. 15. Mandal A, Gote V, Pal D, et al. Ocular pharmacokinetics of a topical ophthalmic nanomicellar solution of cyclosporine (Cequa®) for dry eye disease. Pharm Res. 2019;36:1-21. 16. Gupta PK, Venkateswaran N. The role of KPI-121 0.25% in the treatment of dry eye disease: penetrating the mucus barrier to treat periodic flares. Ther Adv Ophthalmol. 2021;13:25158414211012797. 17. Dang V. Sizing up anti-inflammatories in dry eye disease: a practical guide for optometrists applying these medications. Rev Optom. 2018;155(4):62. 18. Rhen T, Cidlowski JA. Anti-inflammatory action of glucocorticoids - new mechanisms for old drugs. N Engl J Med. 2005;353(16):1711-23. 19. Venkateswaran N, Bian Y, Gupta PK. Practical guidance for the use of loteprednol etabonate ophthalmic suspension 0.25% in the management of dry eye disease. Clin Ophthalmol. 2022:349-55. 20. Ambroziak AM, Szaflik J, Szaflik JP, et al. Immunomodulation on the ocular surface: a review. Cent Eur J Immunol. 2016;41(2):195-208. 21. Schopf L, Enlow E, Popov A, et al. Ocular pharmacokinetics of a novel loteprednol etabonate 0.4% ophthalmic formulation. Ophthalmol Ther. 2014;3(1-2):63-72. 22. Paton DM. Loteprednol etabonate: a formulation for short-term use in inflammatory flares in dry eye disease. Drugs Today (Barc). 2022;58(2):77-84. 23. Murri MS, Moshirfar M, Birdsong OC, et al. Amniotic membrane extract and eye drops: a review of literature and clinical application. Clin Ophthalmol. 2018;12:1105-12. 24. Clark D, Karpecki P, Salapatek AM, et al. Reproxalap improves signs and symptoms of allergic conjunctivitis in an allergen chamber: a real-world model of allergen exposure. Clin Ophthalmol. 2022;16:15-23. 25. Clark D, Sheppard J, Brady TC. A randomized double-masked phase 2a trial to evaluate activity and safety of topical ocular Reproxalap, a novel RASP inhibitor, in dry eye disease. J Ocul Pharmacol Ther. 2021;37(4):193-9. 26. Clark D, Tauber J, Sheppard J, Brady TC. Early onset and broad activity of Reproxalap in a randomized, double-masked, vehicle-controlled phase 2b trial in dry eye disease. Am J Ophthalmol. 2021;226:22-31. 27. Sheppard JD, Wirta DL, McLaurin E, et al. A water-free 0.1% Cyclosporine A solution for treatment of dry eye disease: results of the randomized phase 2B/3 ESSENCE Study. Cornea. 2021;40(10):1290-7. 28. Wirta DL, Torkildsen GL, Moreira HR, et al. A clinical phase II study to assess efficacy, safety and tolerability of waterfree cyclosporine formulation for treatment of dry eye disease. Ophthalmology. 2019;126(6):792-800. 29. Karpecki, P. Power Trio: Three promising new therapeutics look to dramatically change dry eye disease management. Rev Optom. July 2022. Accessed February 20, 2023. 30. Tauber J, Wirta DL, Sall K, et al. SEECASE study group. A randomized clinical study (SEECASE) to assess efficacy, Safety and tolerability of NOV03 for treatment of dry eye disease. Cornea. 2021;40(9):1132-40. 31. Tauber J, Berdy GJ, Wirta DL, et al. GOBI Study Group.Ophthalmology. 2022:S0161-6420(22)01016-8. 32. Schmidl D, Bata AM, Szegedi S, et al. Influence of Perfluorohexyloctane Eye Drops on Tear Film Thickness in Patients with Mild to Moderate Dry Eye Disease: A Randomized Controlled Clinical Trial. J Ocul Pharmacol Ther. 2020;36(3):154-61. 33. Lemp MA, Crews LA, Bron AJ, et al. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31(5):472-8. 34. Zhang X, M VJ, Qu Y, et al. Dry Eye Management: Targeting the Ocular Surface Microenvironment. Int J Mol Sci. 2017;18(7):1398. 35. Yeu, E, Wirta, DL, Karpecki P, et al. Saturn I Study Group. Lotilaner Ophthalmic Solution, 0.25%, for the Treatment of Demodex Blepharitis: Results of a Prospective, Randomized, Vehicle-Controlled, Double-Masked, Pivotal Trial (Saturn-1). Cornea. August 11, 2022. 36. Zhang AC et al. Ophthalmic Physiol Opt. 2020;40(4):389-432. 37. Fromstein SR et al. Clin Optom (Auckl). 2018;10:57-63. 38. Gonzalez-Salinas R, Yeu E, Holdbrook M, et al. Safety and efficacy of topical lotilaner ophthalmic solution 0.25% for the treatment of Demodex blepharitis: A Pilot Study. J Ophthalmol. 2021;2021:3862684. 39. Beckman KA, Katz JA, Majmudar PA, et al. KPI-121 1% for pain and inflammation in ocular surgery. Pain Manag. 2022;12(1):17-23. 40. Sheppard JD, Comstock TL, Cavet ME. Impact of the topical ophthalmic corticosteroid loteprednol etabonate on intraocular pressure. Adv Ther. 2016;33(4):532-52. 41. Cholkar K, Patel SP, Vadlapudi AD, Mitra AK. Novel strategies for anterior segment ocular drug delivery. J Ocul Pharmacol Ther. 2013;29(2):106-23. |


