25th Annual Dry Eye ReportThe most commonly encountered ocular condition enters the spotlight this month. Inside the May 2024 issue of Review of Optometry, get the scoop on omega fatty acids for dry eye, learn how to match dry eye patients to treatments and find a step-by-step guide to managing OSD. Check out the other dry eye-themed articles featured in this issue:
|
It should come as no surprise that dry eye disease (DED) is a pressing global issue. Just look at these statistics for the United States alone: The prevalence of DED approximately tripled between 2005 and 2012, possibly due in part to increased awareness by patients and clinicians but attributed largely to changes in lifestyle.1 More recent studies have estimated prevalence to be anywhere from 18% to 58%.2-5
DED has a profound detrimental impact on patients’ quality of life.6 Moderate dry eye has been likened to living with conditions such as moderate angina, while severe cases rate as equivalent to a disabling hip fracture.7 Dry eye is the sixth most common presentation (accounting for 5.3%) for ocular medical services in the US.8 An estimated 9.2 million American and 5.2 million British individuals suffer from moderate-to-severe DED.9,10 The economic burden to the US healthcare system was estimated over a decade ago to be approximately $3.84 billion annually, while the overall societal cost was around $55.4 billion.8,11
None of these numbers are meant to scare but rather to remind that this condition should not be dismissed as a trivial inconvenience. Diagnosing dry eye and prescribing the correct treatment is crucial for both patients and doctors alike. DED’s pervasive impact on global populations has prompted the development of more diagnostic methods and treatments than those found in most other ocular conditions. For this reason, some clinicians may feel overwhelmed by the task, fearful of neglecting some unavailable tool or intervention.
To help, in 2023 the three of us and a number of colleagues worked at the behest of the World Council of Optometry (WCO) to develop a new resource suitable for use by all optometrists, irrespective of any limitations on access to equipment and techniques. Alcon supported the effort and its aims. Below, we will explain the value and clinical use of the result of this collaboration, known as the WCO Alcon Dry Eye Wheel (Figure 1). An online version available here offers an interactive experience with clickable segments revealing additional detail and professional guidance.
Bottom line: You needn’t have the most expensive equipment in your practice to give a proper diagnosis and offer appropriate treatment. With just a few simple diagnostic tools and tests and the Wheel as guidance, you can have the confidence to take on virtually every dry eye patient that walks through your door.
Proper Diagnosis
How can we define what dry eye is? A well-accepted definition, promulgated by the Tear Film and Ocular Surface Society (TFOS), states that, “Dry eye is a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles.”12 That’s been our operating principle since the 2017 TFOS Dry Eye Workshop (TFOS DEWS II) report.
 |
| Click image to enlarge. |
The pathogenesis of DED is driven by both inflammation and damage to the ocular surface.13,14 Tear film instability promotes excessive tear evaporation and hyperosmolarity and exacerbates friction between the eyelids and the ocular surface, resulting in corneal, conjunctival and lid wiper epitheliopathy, which can be observed as ocular surface staining with the addition of ophthalmic dyes. Deterioration in corneal nerves—vital in mediating inflammatory and nociceptive pathways—can also occur, perpetuating ocular surface damage, inflammation and symptoms.15
The tear film is complex in structure and less than one-tenth the thickness of a human hair.16 It’s no wonder that with dry eye being multifactorial in nature, the balance (homeostasis) of the tear film can be easily disrupted. By definition, DED represents a subset of the umbrella term ocular surface disease (diagnosed on the basis of clinical signs). For dry eye to be a confirmed diagnosis, symptoms must also be present. This means that patients with observable clinical signs but no presenting symptoms should not receive a diagnosis of DED.
To that end, it’s vital that clinicians remember to ask questions about how the eyes feel, including visual symptoms. It is recognized that a patient can become symptomatic if the ocular surface is challenged through contact lenses, environmental changes, chronic medication use (e.g., preserved topical drops for glaucoma) or undergoing refractive or cataract surgery.17-19 In these cases of asymptomatic ocular surface disease, the treatment prescribed may be consistent with that recommended for someone with DED; however, the premise for, and potential benefits of, treatment to an asymptomatic patient would be expected to differ, as might the time frame for treatment in line with the planned intervention.
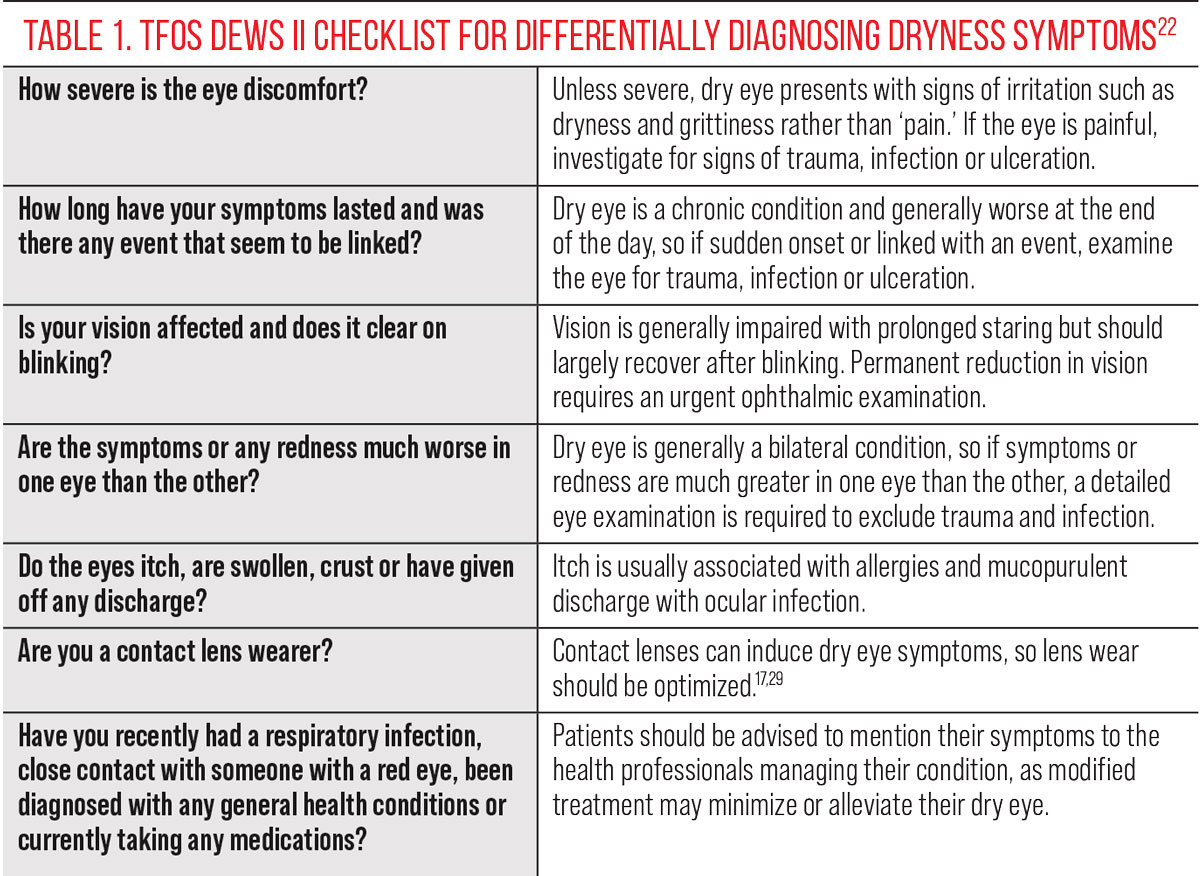
The latter half of the TFOS DEWS II dry eye definition relates to the pathophysiological features specifically associated with DED, to limit confusion with conditions that might masquerade as dry eye, such as ocular allergy or infection. TFOS DEWS II proposed key questions to help inform the differential diagnosis, which may be of particular value to healthcare professionals who have a role in dry eye management and triaging, such as general practitioners and pharmacists.13,20,21
Accurate diagnosis is critical. For patients, it initiates access to the support they need; for practitioners, it enables consistency in approach and offers an evidence-based management plan. Defining diagnostic criteria is not without challenges, though. The diagnostic accuracy of a test should be compared to a “gold standard,” but no such definitive test exists in the clinical setting; therefore, the dry eye diagnostic gold standard needs to defined via expert consensus based on the best available scientific evidence.22
Approaches to setting diagnostic criteria based on an assumed dry eye prevalence are understandably flawed when this prevalence in itself is based on outcomes from a range of non-evidence-based and non-consensus-derived diagnostic criteria.23 Increasing the number of tests can increase the sensitivity and specificity of a diagnosis relative to a pre-defined gold standard but decreases the practicality of making a firm diagnosis due to the testing time and cost burden, which can be counterproductive.22,23 An additional consideration in testing is the risk of induced reflex tearing distorting the assessment, as observed in clinical techniques that destabilize the natural tear film or irritate the eye, such as instillation of fluorescein dye or application of the Schirmer test.24-26 Most importantly, unity in diagnostic testing is critical for the reasons highlighted, regardless of a practitioner’s preferred tests.
 |
|
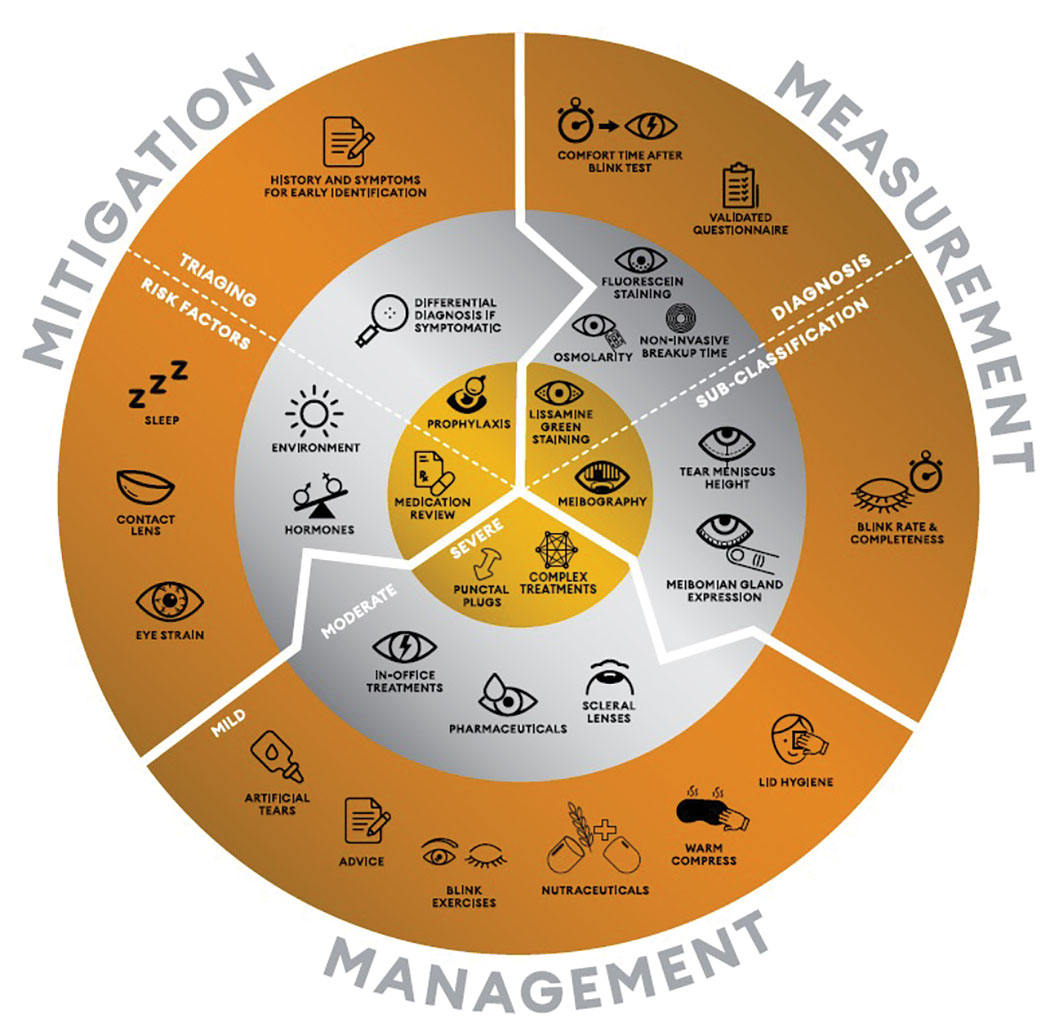
The WCO Alcon Dry Eye Wheel distills much of the TFOS DEWS II report's clinical guidance into a simple conceptual model to follow. Click image to enlarge. |
Dry Eye Wheel
The WCO Alcon Dry Eye Wheel is organized around two categorical frameworks. First, the doctor’s clinical responsibilities are divided into three areas—mitigation, measurement and management—arranged in a circular form with arrows indicating that these efforts should be considered as a continuum. The second framework categorizes the work of DED care into three levels—simple, intermediate and advanced—and represents these, visually, as a series of concentric rings. Thus, you can think of the WCO Alcon Dry Eye Wheel as a 3x3 grid in a more dynamic form. Subdivisions add additional nuance to understanding the mitigation and measurement components, and it’s important to note that all entries should be considered by the healthcare practitioner in relation to the ocular surface health, general health, environment and lifestyle of their individual patients.
Let us now consider the Wheel’s components in more detail, with particular emphasis on the outermost ring, as that is the most broadly applicable.
The Outer Ring
This level of care, denoted in bronze, reflects the most widely accessible strategies and represents the simplest level to master. Within it, the three components of care comprise the following:
• Mitigation. This clinical responsibility requires thorough history and symptom-taking to identify any ocular symptoms, when and for how long they occur and any relevant inciting incident, such as trauma to the eye. The other pressing aspect of mitigation is evaluation of each patient’s risk factors, particularly those that could be modified, such as sleep duration (e.g., less than six hours a night increases the odds of having dry eye), contact lens wear (where mitigation strategies to improve comfort have previously been published) or digital screen use, recognizing that digital eye strain is now very common and the evidence-based effectiveness of treatment strategies has recently been reported.17, 20, 27, 28-30
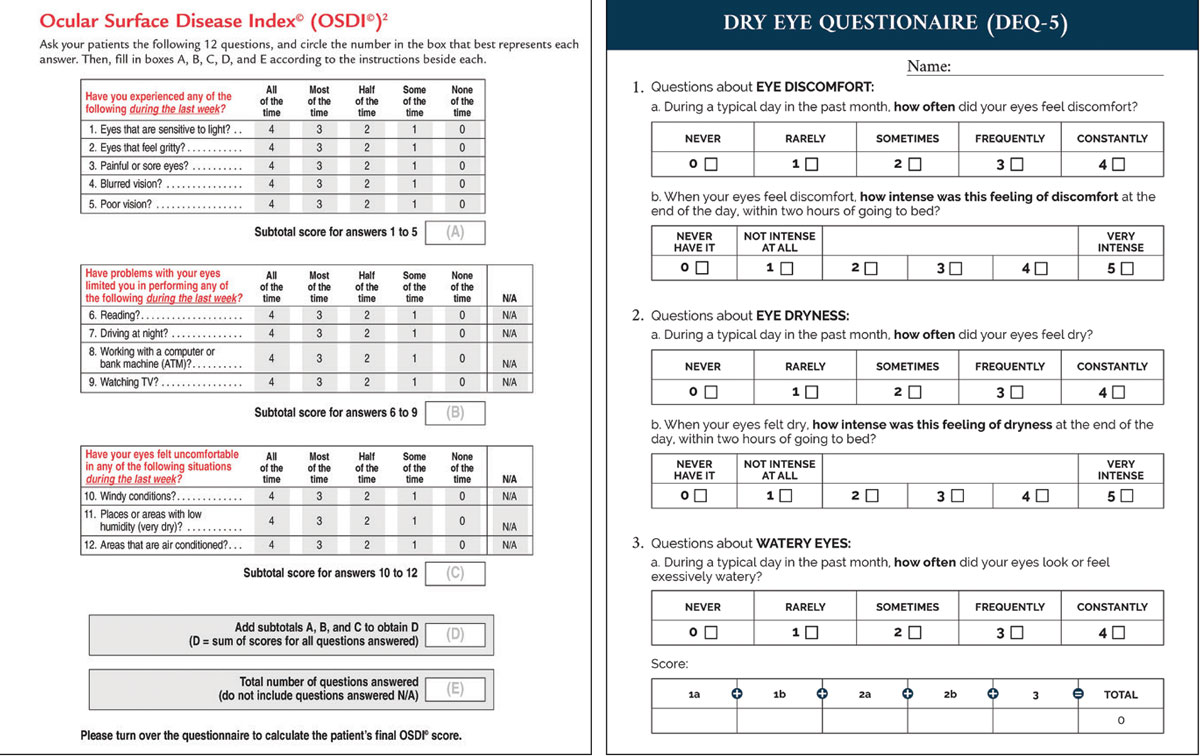
• Measurement. The chief task here is to quantify both symptoms and signs of dry eye. The former can be documented by using the Ocular Surface Disease Index (cut-off ≥13) or 5-Item Dry Eye Questionnaire (cut-off ≥6) based on the TFOS DEWS II diagnostic criteria.22 For the latter responsibility—documenting signs—establishing how long after a blink a patient remains comfortable with their eyes open has been shown to serve as a simple and rapid proxy for tear film stability assessment. This offers sensitivity of 71% and specificity of 90% compared to the full diagnostic workup when using a cut-off of <10 seconds in combination with meeting the symptoms criteria.31 In terms of subclassification, evaporative dry eye impacts blink rate and completeness, which can be easily observed surreptitiously during history and symptom-taking.32
• Management. These recommendations align with the TFOS DEWS II stepwise treatment approach for DED and include advice about relevant risk factors and the impact of the patient’s lifestyle, the use of artificial tears (recognizing that effectiveness depends on the composition chosen for the patient), blink exercises—particularly for those demonstrating partial blinking—nutrition, warm compresses and lid wipes (avoid use of baby shampoo for lid cleansing, as it risks promoting ocular inflammation).33,34
Favorable responders to artificial tears typically report a reduction in symptoms within one month.45 In studies with assessment up to six months, regular ongoing use of artificial tears can result in improved ocular signs over the ensuing months, highlighting the need for eyecare practitioners to encourage good compliance by promoting the use of the drops on an ongoing basis, at least four times a day.45 A global survey of current practice in the management of DED showed that these simple treatments are recommended by practitioners across virtually the full range of dry eye severities and subtypes.46
Middle Ring
This level—the silver ring—incorporates additional aspects to mitigation such as careful differential diagnosis (Table 1) and delves deeper into more complex aspects such as a patient’s environment and the impact of hormones on their dry eye-related symptoms.18,47 Diagnostic measurements requiring a slit-lamp biomicroscope or specialist dry eye instrumentation include noninvasive break-up time assessment, epithelial fluorescein staining (corneal, bulbar and lid margin conjunctival staining at the lid wiper zone) and tear osmolarity. Bear in mind that lid wiper epitheliopathy (a presumed marker of cellular stress) presents much earlier than corneal staining in the natural history of DED development.45
Tests used to subtype aqueous-deficient dry eye include noninvasive tear meniscus height measurement (where less than 0.2mm is indicative of aqueous deficiency) and inspection of meibum secretions.22 Patients with evaporative dry eye commonly present with meibum that is no longer clear but is cloudy, viscous or unable to be expelled by lid expression.48 Other management options for patients where the bronze outer level management and therapy approaches do not offer sufficient relief include pharmacological approaches, in-office light, heat and massaging therapies, and the fitting of scleral lenses to protect and promote hydration of the ocular surface.33
Central Ring
More advanced mitigations, shown in the central gold circle of the WCO Alcon Dry Eye Wheel, include prophylaxis discussions with a patient, such as those who are heavy gamers and smokers, and a possible medication review to limit iatrogenic effects.19,49 Multidisciplinary interaction with the patient’s medication prescribers may be warranted to consider options with less impact on the ocular surface health. Lissamine green staining, both on the bulbar conjunctiva and lid margin, is diagnostic for dry eye, but it should be noted that not all brands stain the ocular surface well.50
Meibography is a useful prognostic test that allows meibomian gland morphology and dropout to be visualized, with remaining gland length in particular associated with gland functionality, which can help inform appropriate treatment selection.51 Viewing the images together with patients can serve as an excellent communication tool and aid with treatment compliance, particularly when considering prophylactic measures for those with less marked symptoms.
Finally, more complex management includes topical therapeutics, punctal plugging, biologic agents (including autologous serum or amniotic membrane) or surgical techniques, but these are required by only a small proportion of patients with dry eye.33
 |
|
The OSDI and DEQ surveys collect vital information that can help clinicians assess dry eye symptoms at baseline and then track response to treatment. Click image to enlarge. |
Takeaways
With an aging population, alongside challenges presented to the ocular surface by modern lifestyles, dry eye is a rapidly growing burden on individual patients and the healthcare system as a whole. Offering strategies to help manage DED is a duty of care for the eyecare professional, and can easily be accomplished with just a few simple tools at your disposal. Standardized diagnosis across the world is critical to ensure consistency in patient messaging and to avoid under-recognition and inadequate management of DED. The Dry Eye Wheel offers a practical, interactive tool highlighting, to all optometrists who encounter individuals with dry eye, ways in which they can get involved to improve quality of life for affected patients.
The authors thank the WCO and Alcon for recognizing the need for a globally applicable dry eye mitigation, measurement and management tool, and supporting its conceptualization and development.
Dr. Wolffsohn is a professor at the School of Optometry and Vision Science, College of Health and Life Science at Aston University in the United Kingdom. He is the academic Chair of the British Contact Lens Association, having been a past president and is on the executive of TFOS. Dr. Wolffsohn is also a Diplomate with Cornea, Contact Lenses and Refractive Technologies. He is a founder of Aston Vision Sciences, Eyoto and Wolffsohn Research Limited. His research team has received research support or lectureship honoraria from 3M, AOS, Alcon, Allergan, Bausch + Lomb, BCLA, CooperVision, CSIDryEye, DopaVision, Essilor, Espansione, International Myopia Institute, Johnson & Johnson Vision, Rayner, M2C Pharmaceuticals, Medmont, Novartis, NuVision, Santen, Scope Ophthalmics, SightGlass, Théa, Topcon and The Eye Doctor. Dr. Craig is a professor in the Department of Ophthalmology at the University of Auckland in New Zealand, where she heads the Ocular Surface Laboratory. She has served as vice chair of TFOS DEWS II and chair of the TFOS Lifestyle Workshop. The Ocular Surface Laboratory has received research support or lectureship honoraria from Alcon, Azura Ophthalmics, Resono Ophthalmic, Photon Therapeutics, Topcon and TRG Natural Pharmaceuticals. Dr. Jones is a professor at the School of Optometry and Vision Science, University Professor and Director of the Centre for Ocular Research & Education (CORE) at the University of Waterloo in Ontario, Canada. CORE has received research support or lectureship honoraria from Alcon, Azura Ophthalmics, Bausch Health, CooperVision, Essilor, Hoya, i-Med Pharma, Integral Biosystems, Johnson & Johnson Vision, Menicon, Novartis, Ophtecs, Oté Pharma, Santen, SightGlass, SightSage, Topcon and Visioneering. Dr. Jones is also a consultant and/or serves on an advisory board for Alcon, CooperVision, Johnson & Johnson Vision, Novartis and Ophtecs.
1. Dana R, Bradley JL, Guerin A, et al. Estimated prevalence and incidence of dry eye disease based on coding analysis of a large, all-age United States health care system. Am J Ophthalmol. 2019;202:47-54. 2. Vidal-Rohr M, Craig JP, Davies LN, Wolffsogn JS. The epidemiology of dry eye disease in the UK: The Aston dry eye study. Cont Lens Anterior Eye. 2023;46(3):101837. 3. García-Marqués JV, Talens-Estarelles C, García-Lazaro S, et al. Systemic, environmental and lifestyle risk factors for dry eye disease in a mediterranean caucasian population. Cont Lens Anterior Eye. 2022;45(5):101539. 4. Wang MTM, Vidal-Rohr M, Muntz A, et al. Systemic risk factors of dry eye disease subtypes: a New Zealand cross-sectional study. Ocul Surf. 2020;18(3):374-80. 5. Cai Y, Wei J, Zhou J, Zou W. Prevalence and incidence of dry eye disease in Asia: a systematic review and meta-analysis. Ophthalmic Res. 2022;65(6):647-58. 6. Hossain P, Siffel C, Joseph C, et al. Patient-reported burden of dry eye disease in the UK: a cross-sectional web-based survey. BMJ Open. 2021;11(3):e039209. 7. Gayton JL. Etiology, prevalence and treatment of dry eye disease. Clin Ophthalmol. 2009:3:405-12. 8. Bradley JL, Ozer Stillman I, Pivneva I, et al. Dry eye disease ranking among common reasons for seeking eye care in a large US claims database. Clin Ophthalmol. 2019;13:225-32. 9. Farrand KF, Fridman M, Ozer Stillman I, Schaumberg DA. Prevalence of diagnosed dry eye disease in the United States among adults aged 18 years and older. Am J Ophthalmol. 2017;182:90-8. 10. Vehof J, Kozareva D, Hysi PG, et al. Prevalence and determinants of dry eye disease in a British female cohort. Invest Ophthalmol Vis Sci. 2013;54:2665. 11. Yu J, Asche CV, Fairchild CJ. The economic burden of dry eye disease in the United States: a decision tree analysis. Cornea. 2011;30(4):379-87. 12. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276-83. 13. Bron AJ, de Paiva CS, Chauhan SK, et al., TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15(3):438-510. 14. De Paiva CS, Corrales RM, Villarreal AL, et al. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Exp Eye Res. 2006;83(3):526-35. 15. Belmonte C, Nichols JJ, Cox SM, et al. TFOS DEWS II pain and sensation report. Ocul Surf. 2017;15(3):404-37. 16. Willcox MDP, Argueso P, Georgiev GA, et al. TFOS DEWS II tear film report. Ocul Surf. 2017;15(3):366-403. 17. Jones L, Efron N, Bandamwar K, et al. TFOS lifestyle: impact of contact lenses on the ocular surface. Ocul Surf. 2023;29:175-219. 18. Alves M, Asbell P, Dogru M, et al. TFOS lifestyle report: impact of environmental conditions on the ocular surface. Ocul Surf. 2023;29:1-52. 19. Gomes JAP, Azar DT, Baudouin C, et al. TFOS lifestyle: impact of elective medications and procedures on the ocular surface. Ocul Surf. 2023;29:331-85. 20. Wolffsohn JS, Lingham G, Downie LE, et al. TFOS lifestyle: impact of the digital environment on the ocular surface. Ocul Surf. 2023;28:213-52. 21. Bilkhu PS, Wolffsohn JS, Tang GW, Naroo SA. Management of dry eye in UK pharmacies. Cont Lens Anterior Eye. 2014;37(5):382-7. 22. Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II diagnostic methodology report. Ocul Surf. 2017;15(3):539-74. 23. Papas EB. Diagnosing dry-eye: which tests are most accurate? Cont Lens Ant Eye. 2023;46(5):102048. 24. Pauk SV, Petricek I, Tomic M, et al. Manual interferometric device for routine non-invasive tear film break-up time assessment. Semin Opthalmol, 2021;36(3):94-102. 25. Cho P, Douthwaite W. The relationship between invasive and noninvasive tear break-up time. Optom Vis Sci. 1995;72(1):17-22. 26. Raj A, Dhasmana R, Nagpal RC. Anterior segment optical coherence tomography for tear meniscus evaluation and its correlation with other tear variables in healthy individuals. J Clin Diagn Res. 2016;10(5):NC1-4. 27. Papas EB, Ciolino JB, Jacobs D, et al. The TFOS International Workshop on Contact Lens Discomfort: report of the management and therapy subcommittee. Invest Ophthalmol Vis Sci. 2013;54(11):TFOS183-203. 28. Ayaki M, Kawashima M, Negishi K, Tsubota K. High prevalence of sleep and mood disorders in dry eye patients: survey of 1,000 eye clinic visitors. Neuropsychiatr Dis Treat. 2015;11:889-94. 29. Ayaki M, Tsubota K, Kawashima M, et al. Sleep disorders are a prevalent and serious comorbidity in dry eye. Invest Ophthalmol Vis Sci. 2018;59(14):DES143-50. 30. Li A, Zhang X, Guo Y, et al. The association between dry eye and sleep disorders: the evidence and possible mechanisms. Nat Sci Sleep. 2022;14:2203-12. 31. Wolffsohn JS, Craig JP, Vidal-Rohr M, et al. Blink test enhances ability to screen for dry eye disease. Cont Lens Anterior Eye. 2018;41(5):421-5. 32. Portello JK, Rosenfield M, Chu CA. Blink rate, incomplete blinks and computer vision syndrome. Optom Vis Sci. 2013;90(5):482-7. 33. Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15(3):575-628. 34. Stapleton FJ, Alves M, Bunya VY, et al. TFOS DEWS II epidemiology report. Ocul Surf. 2017;15(3):334-65. 35. Galor A, Britten-Jones AC, Feng Y, et al., TFOS lifestyle: impact of lifestyle challenges on the ocular surface. Ocul Surf. 2023;28:262-303. 36. Kim M, Lee Y, Mehra D, et al. Dry eye: why artificial tears are not always the answer. BMJ Open Ophthalmol. 2021;6(1): e000697. 37. Pucker AD, Ng SM, Nichols JJ. Over-the-counter artificial tear drops for dry eye syndrome. Cochrane Database Syst Rev. 2016;2(2):CD009729. 38. Semp DA, Beeson D, Sheppard AL, et al., Artificial tears: a systematic review. Clin Optom (Auckl). 2023;15:9-27. 39. Kim AD, Muntz A, Lee J, et al. Therapeutic benefits of blinking exercises in dry eye disease. Cont Lens Anterior Eye. 2021;44(3):101329. 40. Markoulli M, Ahman S, Arcot J, et al. TFOS Lifestyle: impact of nutrition on the ocular surface. Ocul Surf. 2023;29:226-71. 41. Bitton E, Lacroix Z, Leger S. In vivo heat retention comparison of eyelid warming masks. Cont Lens Anterior Eye. 2016;39(4):311-5. 42. Magno MS, Olafsson J, Beining M, et al. Chambered warm moist air eyelid warming devices - a review. Acta Ophthalmol. 2022;100(5):499-510. 43. Borchman D. The optimum temperature for the heat therapy for meibomian gland dysfunction. Ocul Surf. 2019;17(2):360-4. 44. Sung J, Wang MTM, Lee SH, et al. Randomized double-masked trial of eyelid clreansing treatments for blepharitis. Ocul Surf. 2018;16(1):77-83. 45. Craig JP, Muntz A, Wang MTM, et al. Developing evidence-based guidance for the treatment of dry eye disease with artificial tear supplements: a six-month multicentre, double-masked randomised controlled trial. Ocul Surf. 2021;20:62-9. 46. Wolffsohn JS, Trave Huarte S, Jones L, et al. Clinical practice patterns in the management of dry eye disease: a TFOS international survey. Ocul Surf. 2021;21:78-86. 47. Sullivan DA, Rocha EM, Aragona P, et al. TFOS DEWS II sex, gender and hormones report. Ocul Surf. 2017;15(3):284-333. 48. Tomlinson A, Bron AJ, Korb D, et al. The international workshop on meibomian gland dysfunction: report of the diagnosis subcommittee. Invest Ophthalmol Vis Sci. 2011;52(4):2006-49. 49. Muntz A, Turnbull PR, Kim AD, et al. Extended screen time and dry eye in youth. Cont Lens Anterior Eye. 2022;45(5):101541. 50. Delaveris A, Stahl U, Madigan M, Jalbert I. Comparative performance of lissamine green stains. Cont Lens Anterior Eye. 2018;41(1): 23-7. 51. Bilkhu P, Vidal-Rohr M, Trave-Huarte S, Wolffsohn JS. Effect of meibomian gland morphology on functionality with applied treatment. Cont Lens Ant Eye. 2022;45(2):101402. |

