 |
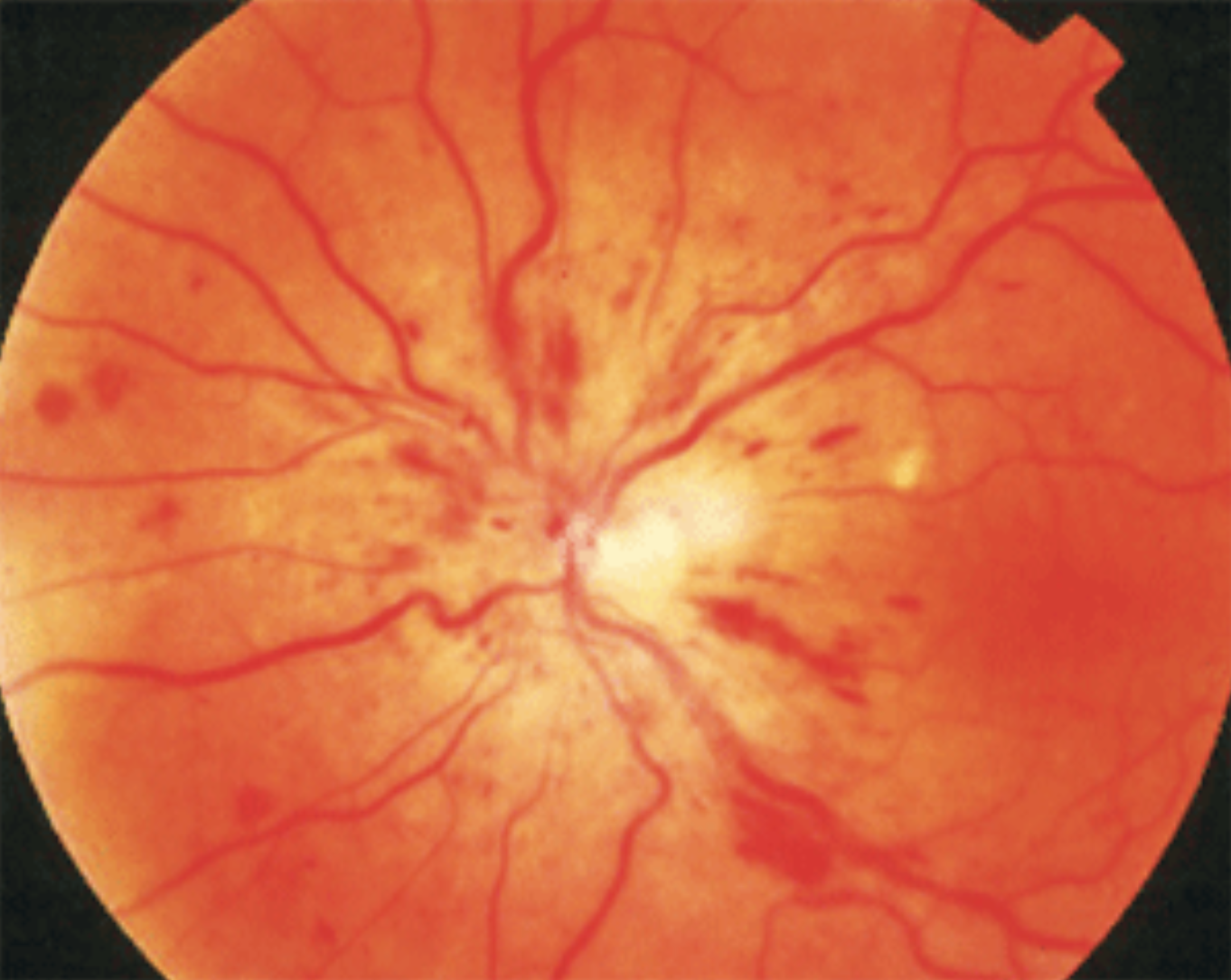
Among 123 patients with CRVO, the cumulative probability of developing NVG was 26.8%, which occurred 507 days after CRVO diagnosis, on average. Photo: Joesph Sowka, OD. Click image to enlarge. |
Approximately 8% of patients with central retinal vein occlusion (CRVO) develop neovascular glaucoma (NVG) within weeks to two years after diagnosis. This likelihood is even greater at 20% for patients with ischemic CRVO, defined as ≥ 10-disc diameter areas of nonperfusion on fluorescein angiography. Despite these concerning odds, clinical predictors for the progression of NVG in this population are largely understudied. To help identify which patients may need especially close monitoring, researchers recently explored potential risk factors for NVG development in CRVO patients over a two-year follow-up period.
The study reviewed 1,545 patients with CRVO seen at a single hospital in Taiwan over 15 years. Of these, 123 patients met the following inclusion criteria and were included in the study: (1) acute CRVO within three months, (2) ocular neovascularization at initial presentation, (3) no treatment received at the time of CRVO and (4) at least bimonthly follow-ups for a minimum of two years.
In the overall follow-up period, the cumulative probability of developing NVG was 26.8% (33 of 123 cases). The average interval between the onset of CRVO and NVG was 507 days.
After controlling for other variates, the analysis indicated that diabetes mellitus, older age and poor vision were significant independent risk factors for developing NVG in CRVO patients. Conversely, the following factors had no significant impact on the likelihood of developing NVG: presence of macular edema, increased central macular thickness, cardiovascular events, history of glaucoma and early PRP within three months.
Diabetes, the first of the three risk factors identified, “is notorious for its microvascular complications due to the destruction of healthy retinal vasculature,” the study authors explained in their paper, appearing in Ophthalmic Epidemiology. “One of the possible explanations is that decreased capillary perfusion stemming from leukocyte aggregation in retina vessels, which leads to the release of hypoxia-inducible factor-1α and VEGF, results in promoting uncontrolled angiogenesis,” they wrote.
To help explain how the second risk factor, age, increases NVG risk in CRVO, the researchers relay that “it has been proven that microvascular structure loses their integrity and complexity with age and lead to reduced functionality.” In fact, for every one year older, there was a corresponding 1.07-times greater risk of developing NVG. The authors proposed that “with less healthy vasculature, we speculated that the aging process decreases protection against retinal ischemia, which may be the cause of developing NVG in CRVO patients.”
Lastly, the authors elaborated on the finding that poor vision increases the likelihood of NVG in CRVO. They pointed out that worse visual acuity (VA) at baseline has been shown in previous research to be a strong predictor of increased risk of neovascularization of iris, angle neovascularization and even NVG. The findings of those former studies suggested that “poor VA was related to the larger non-perfusion area caused by acute CRVO, while severe retinal ischemia is prone to inducing neovascularization,” the current study authors noted in their paper. “Despite some studies had demonstrated potential benefits in CRVO patients with worse VA at baseline after anti-VEGF therapy, the tendency to NVG seems inevitable since the severity of ischemic area was already determined at the onset of CRVO and cannot be changed by subsequent treatment.”
There are several limitations of this study, including its retrospective nature, small sample size due to strict inclusion criteria and exclusively Eastern Asian population. Additionally, gonioscopy was not routinely performed in every return visit, leaving room for the possibility that early NVG without elevated intraocular pressure could have been missed. However, the researchers clarified that while this may postpone the detection of NVG, it would not alter the tendency to NVG events.
The findings of this study indicate that patients with CRVO who have comorbidities such as diabetes, older age and worse VA “warrant closer attention and intensive follow-up for the development of NVG,” the authors concluded.
Chou YB, Chang HH, Chiu HI, Chou YJ, Pu C. Risk factors for developing neovascular glaucoma in central retinal vein occlusion: two-year real- world study. Ophthalmic Epidemiology. July 31, 2024. [Epub ahead of print]. |


