 |
|
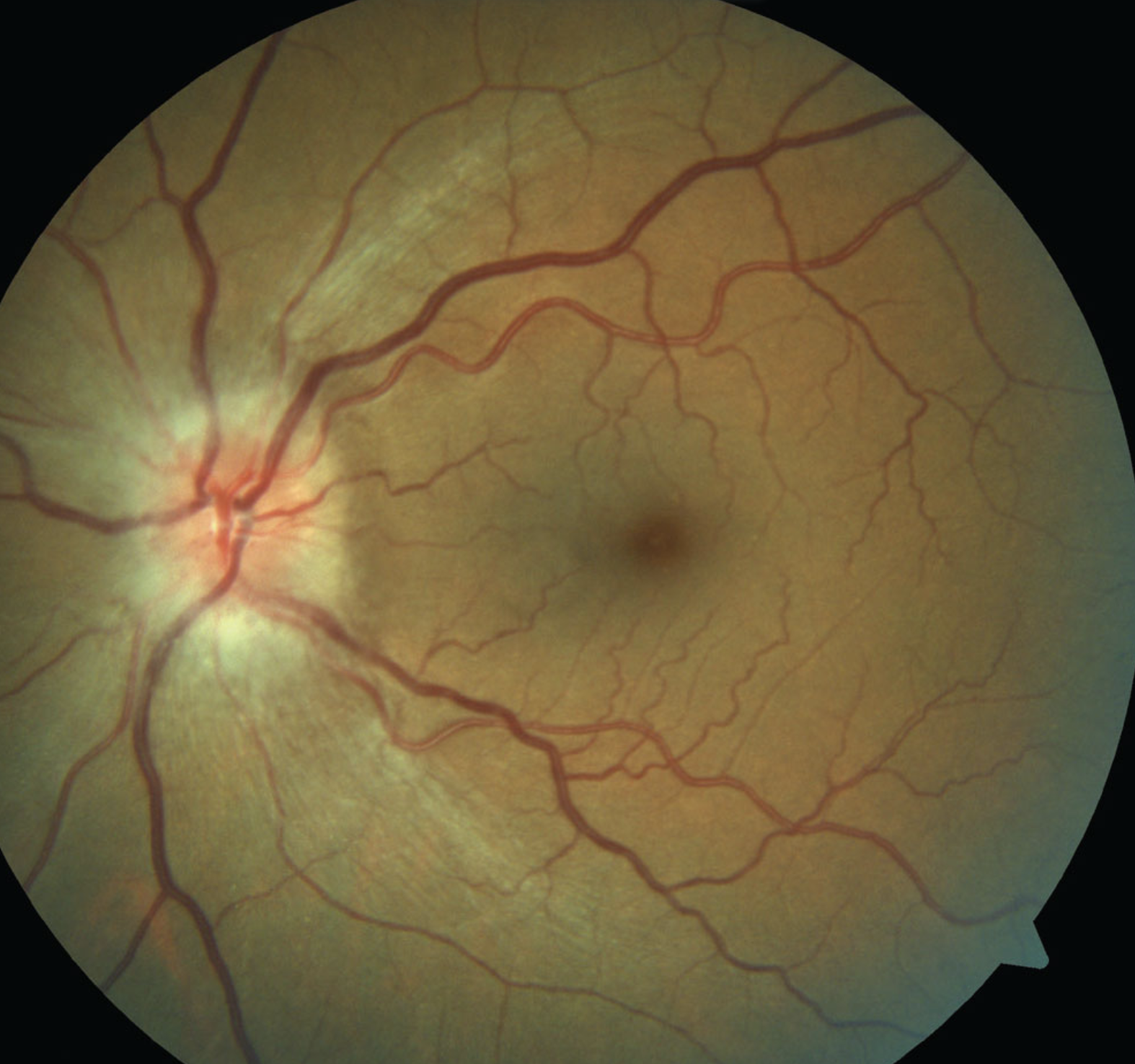
A recent study found an association between the use of semaglutide-a GLP-1 medication for type 2 diabetes and weight loss—and NAION development, though causation remains to be determined. Photo: Michael Trottini, OD, and Candice Tolud, OD. Click image to enlarge. |
Earlier this month, researchers at Harvard University published the results of their recent study, which observed an increased risk of nonarteritic anterior ischemic optic neuropathy (NAION) in patients taking semaglutide for type 2 diabetes or weight loss. Semaglutide, a glucagon-like peptide receptor agonist (GLP-1 RA), is the active ingredient in Ozempic and Wegovy, two medications being prescribed with increasing frequency across the US, adding to a cause for concern about the study's findings.
The American Academy of Ophthalmology (AAOph) and the North American Neuro-Ophthalmology Society recently released a statement responding to the study and commenting on its potential implications for clinical practice. The primary stance of the two organizations is that, while the observed association between semaglutide and NAION of this study is “interesting,” more research is warranted to confirm whether the relationship is causal.
"The type of study conducted here helps identify potential links between GLP-1 treatment and NAION, but it’s not the type of study that can show the treatment caused NAION,” states Andrew Lee, MD, a clinical spokesperson for the American Academy of Ophthalmology and a neuro-ophthalmologist at Houston Methodist Hospital, in a recent Vision Monday article. Until more research is conducted, he says “patients should be aware of this information and, in consultation with their care team, make a careful, informed choice based on their individual risk profile.”
The AAOph and the North American Neuro-Ophthalmology Society offered several other comments and concerns regarding the design and limitations of the study. For one, they point out that prior to its 2017 FDA approval, semaglutide was rigorously studied in multiple randomized controlled trials around the world. Notably, this is the first study to report an association between semaglutide and NAION.
They also noted that subjects in this study were either overweight, obese or had type 2 diabetes, the latter of which is an established risk factor of NAION, with others including heart disease, history of heart attack, high blood pressure and sleep apnea. However, the study authors did assert that they controlled for these potential confounders in their analysis.
Another potential limitation of the study according to the AAOph and the North American Neuro-Ophthalmology Society was that all patients included were seen at Massachusetts Eye and Ear in Boston. Because the specialty hospital sees a large percentage of NAION patients in the region, this could limit the generalizability of the findings.
The two organizations also point out that semaglutide has previously been linked to other vision changes, such as blurred vision, worsening of diabetic retinopathy and macular complications, though these effects typically subside within three or four months.
Though the AAOph and the North American Neuro-Ophthalmology Society do not recommend that people stop taking semaglutide at this time, they reiterate that further research will help clarify the relationship between the drug and ocular events such as NAION.


