The recent introduction of topical pilocarpine agents for improved near vision in presbyopes has generated interest in a potential new modality of vision correction, but also raised concerns about rhegmatogenous retinal detachment (RRD) risk. A new retrospective study published by American Journal of Ophthalmology examined risk in those using the drug for presbyopia and development of this rare adverse event.
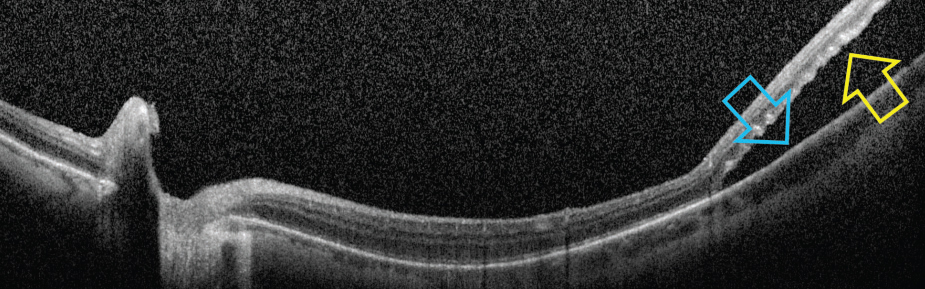 |
| One theory for possible etiology of RRD following pilocarpine is due to ciliary muscle contraction and rotation, which may exert traction and stretching on the peripheral retina and result in in retinal breaks near the ora serrata. Photo: Mohammad Rafieetary, OD. Click image to enlarge. |
An aggregated electronic health records research network was used to identify patients, with the primary study group consisting of individuals over 40 who received topical pilocarpine (1.25% or any dose with exclusion of other indications) for the first time; controls were patients with presbyopia who were started on artificial tears and no history of topical pilocarpine use during the study period. Propensity score matching was employed across both cohorts based on demographics, systemic comorbidities and known RRD risk factors.
After this matching, it was found that the three-month risk of RRD was significantly higher in the pilocarpine group (0.53%) vs. control group (0.25%). This disparity was echoed in six-month risk data as well, with elevated levels remaining in the study group of 0.60% vs. just 0.31% for controls. At one year, RRD risk increased in the pilocarpine group to 0.78% and stayed relatively stable at 0.33% in the control group. Using a model, pilocarpine was found to pose a 3.14-fold increased risk of RRD when compared with controls, and this was after adjusting for demographics or comorbidities. Additional risk factors of male sex, myopia, vitreous degeneration, lattice degeneration and pseudophakia were identified.
Heightened RRD risk in the pilocarpine group was over twofold greater in the first three months after therapy initiation and an adjusted hazard ratio of 3.14 at a one-year endpoint, with the increased risk persisting throughout the first year of use. In those with myopia, risk was approximately twofold; with lattice degeneration risk was roughly fourfold, vitreous degeneration was about twofold and pseudophakia was threefold also in the first year after initiation.
The authors stress the importance of these results, as the GEMINI I and II Phase III clinical trials of Vuity did not report any RRD cases with 1.25% pilocarpine; however, both included only 750 patients, a level not sufficient to reflect uncommon adverse events, the researchers say. As well, those with history of cataract surgery, myopia of at least 4.00D or pre-existing ocular conditions that could impact the safety of the participant were excluded, thus not reflecting real risk when used outside of a study setting.
The authors write in their paper that “these findings underscore the importance of conducting a thorough ocular examination and providing comprehensive patient counseling regarding the potential risks associated with topical pilocarpine therapy.” Patients should be educated about warning symptoms of RRD such as flashes of light, floaters or vision loss. They also recommend those who start pilocarpine with pre-existing risk factors of myopia, lattice degeneration or pseudophakia undergo a comprehensive dilated retinal exam before starting treatment.
| Click here for journal source. |
Elhusseiny AM, Chauhan MZ, Jabbehdari S, et al. Using real-world data to assess the association of retinal detachment with topical pilocarpine use. Am J Ophthalmol. November 5, 2024. [Epub ahead of print]. |


