More than 15 treatments are under investigation for geographic atrophy (GA) at the moment. Though last year saw the first two drug approvals for GA, these therapies have relatively modest anatomic efficacy, require intravitreal injection and increase the risk of neovascular AMD, signaling the need for more desirable treatment approaches.
One candidate for GA undergoing clinical trials is oral minocycline, a microglial inhibitor. This investigative drug recently wrapped up its Phase II prospective, single-arm, 45-month nonrandomized controlled trial, the outcomes of which were detailed in a recent JAMA Ophthalmology study. The findings showed that oral minocycline 100mg is likely not associated with a slower rate of enlargement of GA in AMD.
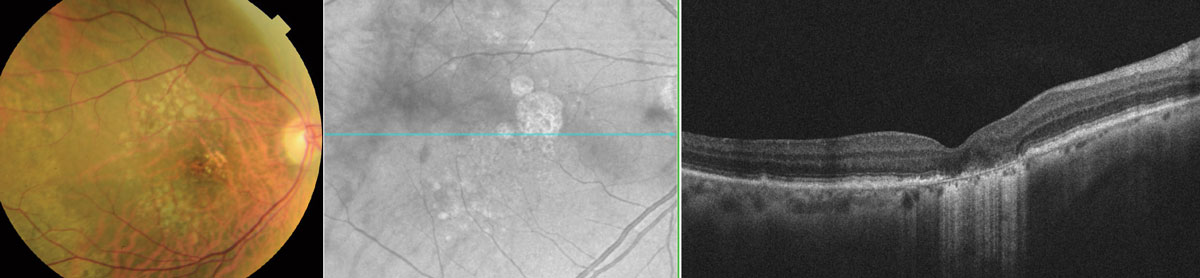 |
|
At a dose of 100mg, oral minocycline appeared to have no clinically meaningful treatment effect on GA in AMD among 37 Phase II trial participants. Photo: Wendy Harrison, OD, PhD. Click image to enlarge. |
Thirty-seven patients with a mean age of 74.3 years were enrolled in the trial. The study's primary outcome measure, assessed at week 33, was the difference in the rate of change of square root GA area on fundus autofluorescence between the 24-month treatment phase and a nine-month run-in phase, which took place before treatment was initiated.
After comparing data from the run-in phase vs. the treatment phase, researchers concluded that minocycline did not significantly decrease GA enlargement over the 24 months of the trial. The mean square root GA enlargement rate among study eyes was 0.31mm per year during the run-in phase and 0.28mm per year during the treatment phase, for a minuscule difference of -0.03mm per year between the two phases.
The mean difference in rate of change between the two phases was also not significantly different; for visual acuity, the difference was 0.2 letter score per month, and for subfoveal retinal thickness, the difference was 0.7μm per month.
Regarding treatment-emergent adverse events, a hefty total of 129 occurred among the 32 participants, though importantly, none were considered severe. Forty-nine adverse events (38%) were related to minocycline (none were ocular), including elevated thyrotropin level (15 cases) and skin hyperpigmentation/ discoloration (eight cases).
To sum up all these data points, the researchers wrote in their JAMA Ophthalmology paper on the study that oral minocycline “was not associated with a definitive decrease in rates of decline in BCVA, low luminance visual acuity or subfoveal retinal thickness,” and that, “no consistent signal of a clinically meaningful treatment effect was apparent, either for structural or visual acuity endpoints.”
The study authors attempted to theorize reasons for the treatment’s lack of effect. The most likely explanation, they noted, is that “minocycline has no substantial association with slowing GA enlargement. It is possible that minocycline did not inhibit microglia consistently at the site of disease progression, potentially related to dose, bioavailability, or pharmacokinetics,” they wrote. It’s also possible that a type II error occurred, given the small number of participants in the trial, though the authors argued in their paper that “this scenario seems unlikely; a sample size of 17 participants was calculated as necessary for 90% power.”
In conclusion, the Phase II trial for oral minocycline as a treatment for GA in AMD did not observe a significant clinical treatment effect, at least not at the tested dose of 100mg. While the drug was relatively tolerable, it also led to a high number of mild or moderate systemic adverse events. “However,” the authors wrote, “given the potential disadvantages of existing therapeutic approaches, additional strategies remain desirable. It may be necessary to elucidate more clearly the pathogenetic mechanisms underlying GA incidence and progression to develop therapies that target the underlying disease processes.”
Keenan TDL, Bailey C, Abraham M, et al. Phase 2 trial evaluating minocycline for geographic atrophy in age-related macular degeneration: a nonrandomized controlled trial. JAMA Ophthalmol. March 14, 2024. [Epub ahead of print]. |


