Since the launch of Eylea (aflibercept 2mg, Regeneron) over a decade ago, retina specialists and comanaging optometrists and ophthalmologists have relied heavily on this anti-VEGF agent for treating patients with neovascular age-related macular degeneration (nAMD) and other retinal disorders. However, the requirement of injections every one to two months can place a considerable burden on patients and practices, which led the company to develop a high-dose formulation (Eylea HD, 8mg) that can extend the maintenance interval by several months. The new drug gained FDA approval last summer based on the positive results of two clinical trials, Photon and Pulsar, which demonstrated non-inferiority and clinically equivalent vision gains at 48 weeks with eight-, 12- and 16-week dosing regimens after the three initial monthly doses.
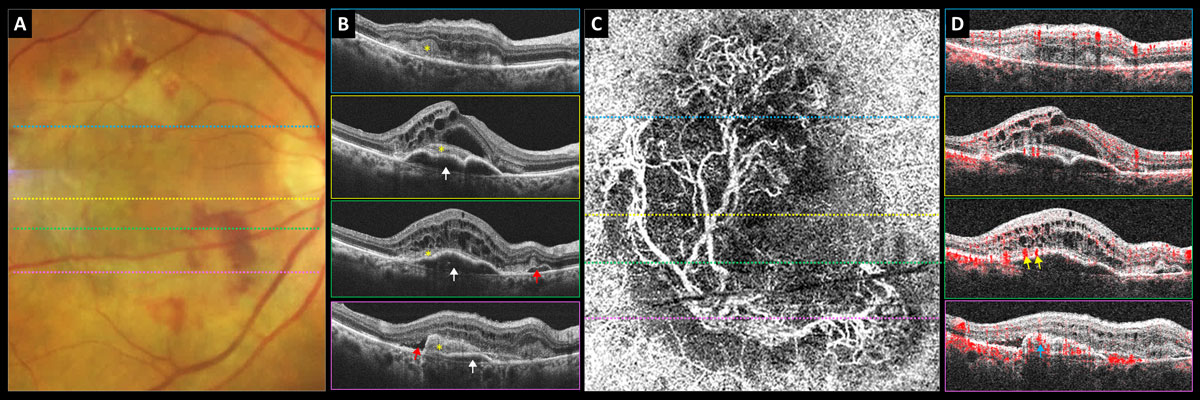 |
| The 96-week results of the Pulsar Phase III trial for Eylea HD demonstrate that high-dose aflibercept can offer nAMD patients stable vision with fewer injections than the 2mg formulation. Photo: Carolyn Majcher, OD. Click image to enlarge. |
Last week at ARVO in Seattle, researchers presented the 96-week results of the Pulsar trial, which evaluated the safety and efficacy of Eylea HD for nAMD. Similar to the 48-week outcomes, the drug showed that patients receiving high-dose aflibercept every 12 or 16 weeks—or longer in year two—maintained similar BCVA gains and had a safety profile compared with those treated with the 2mg formulation every eight weeks.
In the double-masked Pulsar trial, patients were randomly assigned 1:1:1 to receive aflibercept 8mg every 12 or 16 weeks (8q12 [n=335] or 8q16 [n=338]) or aflibercept 2mg every eight weeks (2q8 [n=336]), each after three initial monthly injections. From week 52 to week 96, dosing regimens for the high-dose aflibercept groups could be extended as needed based on study criteria.
Here are the results recorded at week 96 of the Pulsar trial:
- Seventy-five percent of 8q12 patients and 70% of 8q16 patients maintained ≥12- and ≥16-week dosing intervals.
- In the combined aflibercept 8mg arm, 47% of patients who completed 96 weeks had dosing intervals of ≥20 weeks at week 96, and 28% had a 24-week dosing interval.
- No new side effects were identified with aflibercept 8mg.
“In participants who received two years of aflibercept 8mg, almost half could have the time between injections increased to at least five months,” the ARVO presenters wrote in their abstract. Accordingly, “These findings suggest that aflibercept 8mg may reduce the need for frequent injections in people with wet AMD,” they concluded.
Original abstract content @2024 Association for Research in Vision and Ophthalmology.
Sivaprasad S, Korobelnik JF. BCVA gains with aflibercept 8 mg maintained through week 96 in PULSAR with extended treatment intervals in patients with nAMD. ARVO 2024 annual meeting. |


