 |
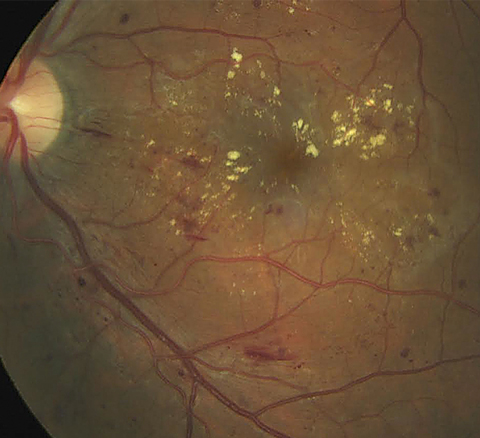
| The DEX implant may offer patients with DME a treatment option that requires up to 11 fewer injections per year than anti-VEGF. Photo: Erik Hanson, MD. Click image to enlarge. |
For nearly the last two decades, the standard of care in the management of diabetic macular edema (DME) has been intravitreal anti-VEGF therapy; however, the patient burden from this kind of treatment can be high, as it typically involves frequent injections and visits to the office, contributing to noncompliance.
An intravitreal implant developed by Allergan as an alternative to anti-VEGF therapy may offer an alternative first-line therapy option for the treatment of sight-threatening DME. The Ozurdex implant (dexamethasone 0.7mg) releases corticosteroid into the eye over time and takes away the need for and risks associated with monthly injections. To evaluate its safety and effectiveness, a research team in Australia recently found that treatment-naive eyes with DME treated with Ozurdex experienced positive outcomes and no new safety concerns.
A total of 200 eyes of patients 18 years or older from 25 ophthalmology clinics in Australia were examined. Study participants had either pseudophakic or phakic eyes scheduled for cataract surgery that were treatment-naive (n=57) or nonresponsive to anti-VEGF therapy. After an initial Ozurdex injection, patients returned for reinjection at intervals of at least 16 weeks. At every appointment, including the baseline visit and the final visit at 52 weeks, best-corrected visual acuity (BCVA) and central subfield retinal thickness (CRT) were recorded.
The mean number of Ozurdex injections over the 52-week study period was 2.4, with 23.6% of patients receiving one injection, 21.8% receiving two, 40% receiving three and 14.5% receiving four. The mean interval for injections was 136 days. For treatment-naive eyes treated with Ozurdex, mean BCVA increased by 3.4 letters and mean CRT decreased by 89.6µm from baseline to week 52. Even at week 16 with one Ozurdex injection, the change in mean CRT from baseline was -55.8µm. A significant change in mean BCVA from baseline was observed at weeks six, 16 and 24.
The most common adverse event observed in eyes with the implant was increased intraocular pressure (IOP). One in five eyes required IOP-lowering medication, and one patient had to discontinue therapy as a result of increased IOP. “It is notable that no cases of elevated IOP recorded during the study required intervention with laser or surgery, similar to what was reported in other investigations of [Ozurdex] in clinical settings with 12-month follow-up,” the researchers wrote in their study. Other than a possible increase in IOP, no serious adverse events were reported among treated patients.
This study can be tacked on to a list of others that have confirmed the safety profile and effectiveness of Ozurdex monotherapy in treating DME, regardless of lens status. The dexamethasone implant offers a promising alternative treatment option to patients with treatment-naive DME and may allow them to receive up to 11 fewer injections per year than with anti-VEGF therapy.
Fraser-Bell S, Kang HK, Mitchell P, et al. Dexamethasone intravitreal implant in treatment-naive diabetic macular oedema: findings from the prospective, multicentre, AUSSIEDEX study. Br J Ophthalmol. July 11, 2021. [Epub ahead of print]. |


