In geographic atrophy (GA), intervention prior to the onset of manifest atrophy may be necessary to achieve better functional outcomes. To help characterize presentations of RPE and outer retinal atrophy (RORA) in GA patients, researchers have settled on two forms: complete RORA (cRORA) is considered to be a region of choroidal hypertransmission of at least 250µm, as well as evidence of overlying photoreceptor degeneration in the absence of a scrolled RPE or other signs of an RPE tear, while incomplete RORA (iRORA) was defined similarly, aside from not fulfilling the 250µm region.
 |
|
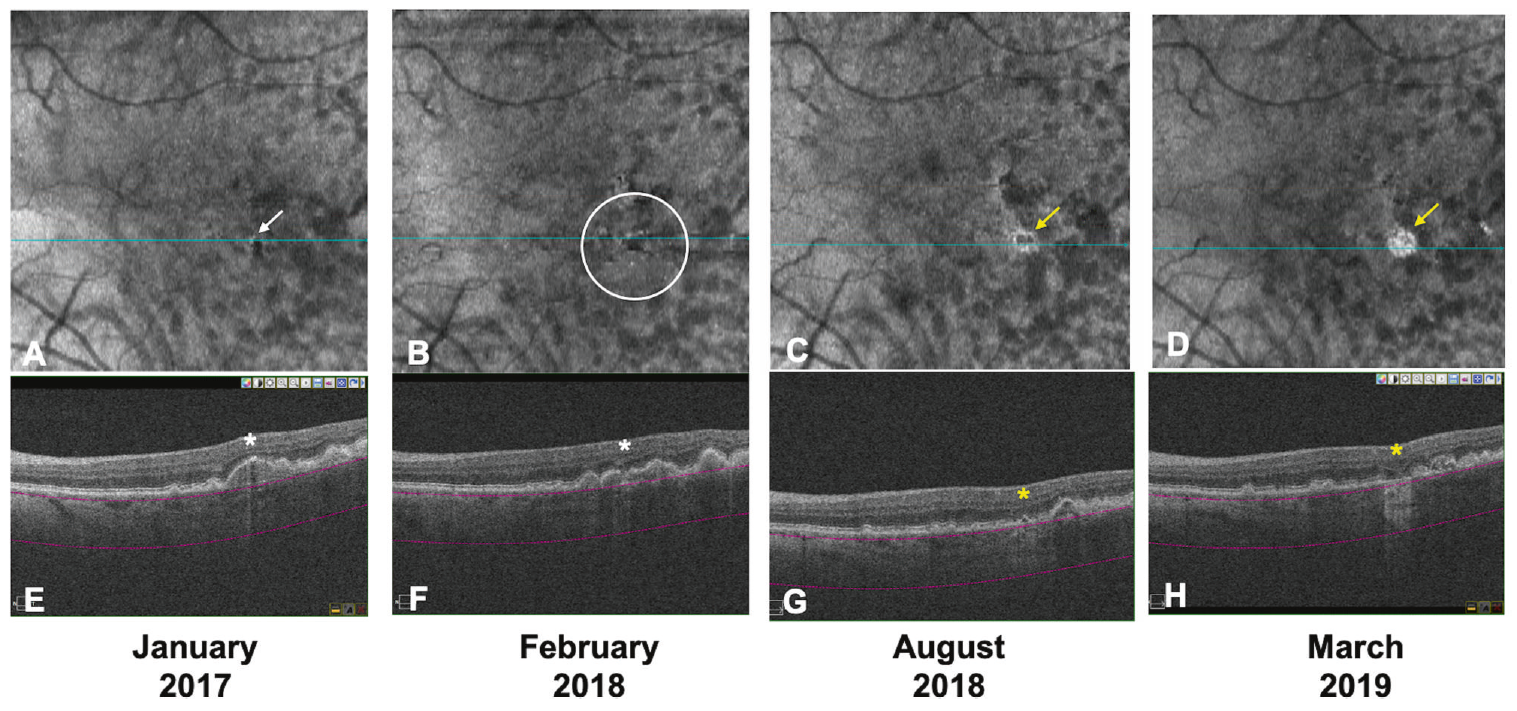
Over two years of follow-up, the majority of eyes with no hypertransmission defects (hyperTDs) at baseline (74.5%) were stable, while eyes with defects (87.5%) were more likely to progress (ORs ranged from 16.1 to 26.4). This image from the study shows an example of an eye with hyperTD with progression: A single small hyperTD (white arrow) is detected on the baseline en face OCT (A). The corresponding horizontal OCT B-scan (E) shows choroidal hypertransmission and a small region of RPE disruption, but no overlying photoreceptor degeneration (white asterisk). The criteria for iRORA are not met at baseline on OCT. Fourteen months from baseline, multiple small hyperTDs (white circle) are evident with a progression to iRORA (B and F). Six months later (20 months from baseline), these small hyperTDs coalesced and developed into large hyperTDs with a hyporeflective core (donut; yellow arrow) on the en face OCT (C) and iRORA (yellow asterisk) on the corresponding B-scan (G). At the final follow-up (26 months from baseline), a large hyperTD without a hyporeflective core (yellow arrow) is evident on the en face OCT (D) with cRORA (yellow asterisk) on the corresponding B-scan (H). This eye is deemed a progression eye. Click image to enlarge. (Photo: Nanegrungsunk O, et al. Eye (Lond). September 15, 2024) |
Defining iRORA and cRORA on OCT may offer potential new biomarkers and endpoints to monitor the progression to atrophy in a more granular fashion. To address these concerns, some researchers have suggested focusing on choroidal hypertransmission. With a choroidal en face image, the hypertransmission appears as discrete regions of hyperreflectivity (termed “hypertransmission defects”) that readily lend themselves to reliable identification and quantification.
A recent study investigated the associations between the presence of various-sized hypertransmission defects and progression to iRORA and in eyes with intermediate age-related macular degeneration (AMD). They found that while most of the intermediate AMD eyes without hypertransmission defects at baseline were likely to remain stable over the next two years, eyes with these defects of any size, especially if there were multiple, are at substantially higher risks for progression to iRORA, cRORA and/or wet AMD. So, these hypertransmission defects may be useful for risk stratification and selection of high-risk patients for early intervention clinical trials for intermediate AMD.
OCT data from consecutive intermediate AMD patients were retrospectively reviewed. All intermediate AMD eyes with or without iRORA (but not cRORA) at baseline were included. Graders evaluated the presence of hypertransmission defects at baseline (small: 63µm to 124µm; medium: 125µm to 249µm; large: ≥250µm in diameter on choroidal en face OCT) and the progression two years later.
Of the 145 eyes that did not develop wet AMD at two years, the eyes that progressed to or developed iRORA or cRORA included 13 eyes (10.7%), five eyes (83.3%), nine eyes (81.8%) and six eyes (85.7%) in the groups with no, small, medium and large hypertransmission defects at baseline, respectively. The odds ratios for progression were 41.6, 37.4 and 49.9 in the small, medium and large hypertransmission defect groups, compared with no defects. Eyes with two or more defects also showed more frequent progression than eyes with one or none (100% vs. 16.4%).
“A hypertransmission defect of any size may be a biomarker for progression to both wet and dry AMD,” the researchers wrote in their paper.
“A possible explanation is that having multiple hypertransmission defects in an eye, irrespective of their size, may reflect more widespread or extensive disease, and thus these eyes are more susceptible for progression,” they added. “Based on our observations, we propose that the presence of small to medium-sized defects and a higher overall number of lesions in an eye may be used to identify an eye that is at high risk for progression and for inclusion in early therapeutic trials.”
| Click here for journal source. |
Nanegrungsunk O, Corradetti G, Phinyo P, et al. Relationship between hypertransmission defect size and progression in eyes with intermediate age-related macular degeneration. Eye (Lond). September 15, 2024. [Epub ahead of print]. |


