 |
Although most clinicians consider dry eye and blepharitis as milder forms of ocular surface disease (OSD), they aren’t to most patients, and masquerading conditions can be disfiguring or even life-threatening. The following rare but significant conditions are ones you don’t want to miss.
 |
|
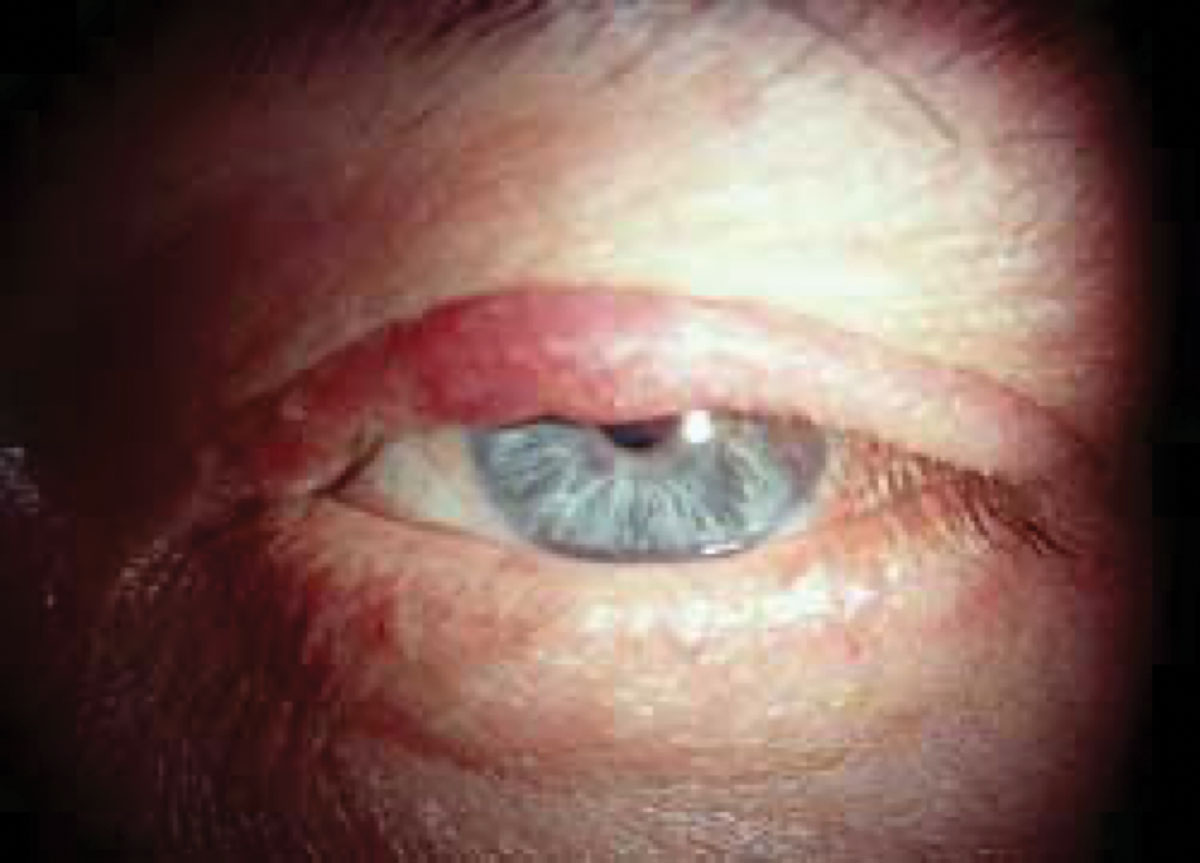
Fig. 1. Sebaceous carcinoma in a patient presenting with a recurrent chalazion. Click image to enlarge. |
Sebaceous Carcinomas
Various terminology exists for this carcinoma, including sebaceous gland carcinoma, sebaceous cell carcinoma and meibomian gland carcinoma. Sebaceous carcinoma is a very aggressive and malignant tumor that originates from anywhere sebaceous glands are found, but is most commonly found on the eyelid from meibomian glands, which have a sebaceous gland origin. The lesion appears as a slow-growing, yellowish mass that is firm but painless to the touch, most commonly on the upper eyelid (Figure 1).1 Often masquerading as a chalazion, this cancerous lesion is quite rare, accounting for about 3% of all malignant tumors and only 0.8% of eyelid tumors.2 Since it is found in meibomian glands, it can also appear as a non-responsive meibomianitis. Signs include madarosis and irregular eyelid margins, and risk factors include radiation exposure, immunosuppression and Muir-Torre syndrome.3 This condition is an autosomal dominant form of hereditary colorectal cancer caused by mutations in the DNA mismatch repair genes.
Individuals with sebaceous carcinoma have a five-year overall survival rate of 78% for localized disease and 50% for metastatic disease.4 If both the upper and lower eyelid are involved or if the lesion is more than 10mm in size, the prognosis is far worse. Once diagnosed, sebaceous cell carcinoma is typically treated via surgical excision and radiotherapy. The lesion, however, tends to be quite infiltrative, therefore it’s not uncommon for a patient with a delayed sebaceous carcinoma diagnosis to have more than 50% of the eyelid removed.5 If not diagnosed, these can metastasize via the lymph nodes to remote sites.
 |
|
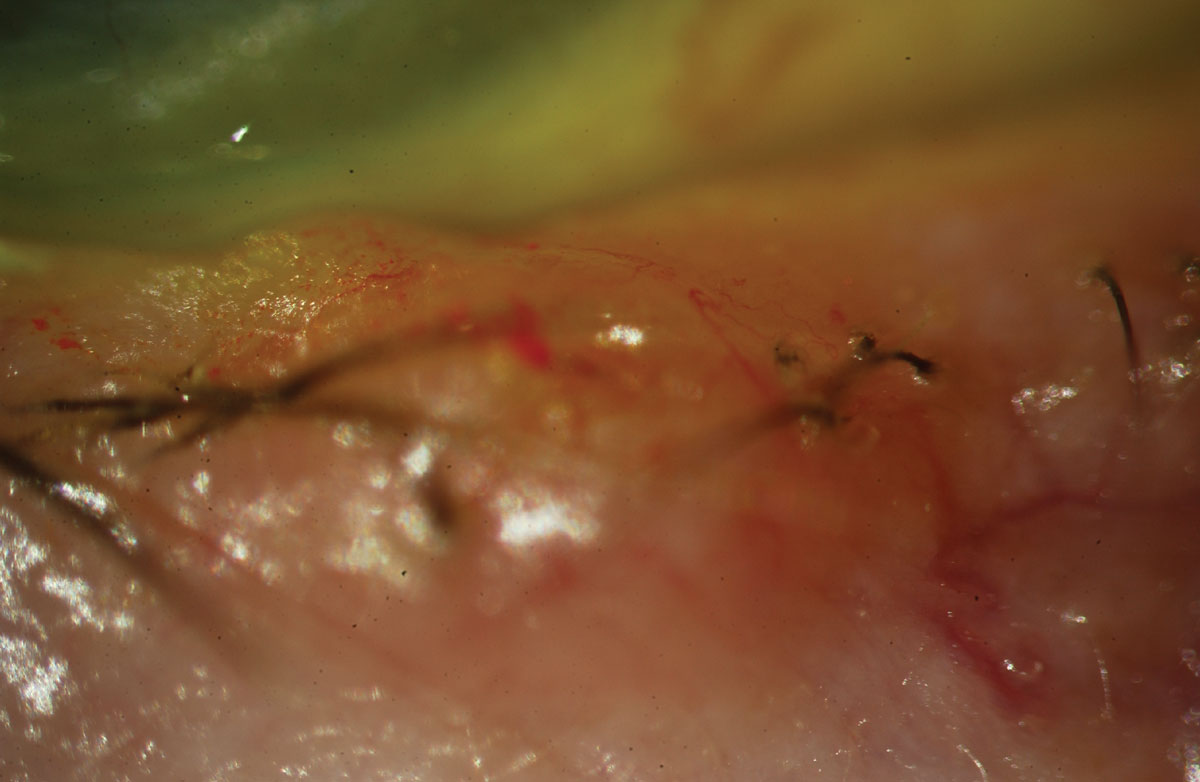
Fig. 2. Basal cell carcinoma with pearly margins and madarosis. Click image to enlarge. |
Basal Cell Carcinomas
A far more common skin cancer often found around the eyelid margins is a basal cell carcinoma (BCC). Studies show BCCs are on the rise.6 The most common sites for BCC include the eyelid margins, the area where nose pads on glasses rest (although nose pads have nothing to do with the formation of a BCC) and behind the ears. Basal cells are responsible for producing new skin cells as old ones die off. Abnormal replication, secondary to risk factors such as UV exposure, can lead to BCC.7 The classic appearance is that of a pearly, translucent margined lesion with an umbilicated center (Figure 2). Hallmark symptoms include repeated crusting and bleeding of the lesion.8 Pain typically occurs as the lesions bleed or especially if they grow to include surrounding tissue like bone and cartilage.
One unique aspect of BCC is that it rarely results in metastasis.9 However, it is extremely invasive in that it grows deep within the tissue. It can result in disfigurement and even be fatal. For this reason, early diagnosis and removal via surgical excision, involving Mohs surgery or other less invasive surgery, is recommended. If caught early and superficial, Imiquimod cream can be applied directly to the lesion for about six weeks. While they can cause significant tissue loss due to depth, they don’t spread like that of a sebaceous carcinoma and are rarely metastatic.6
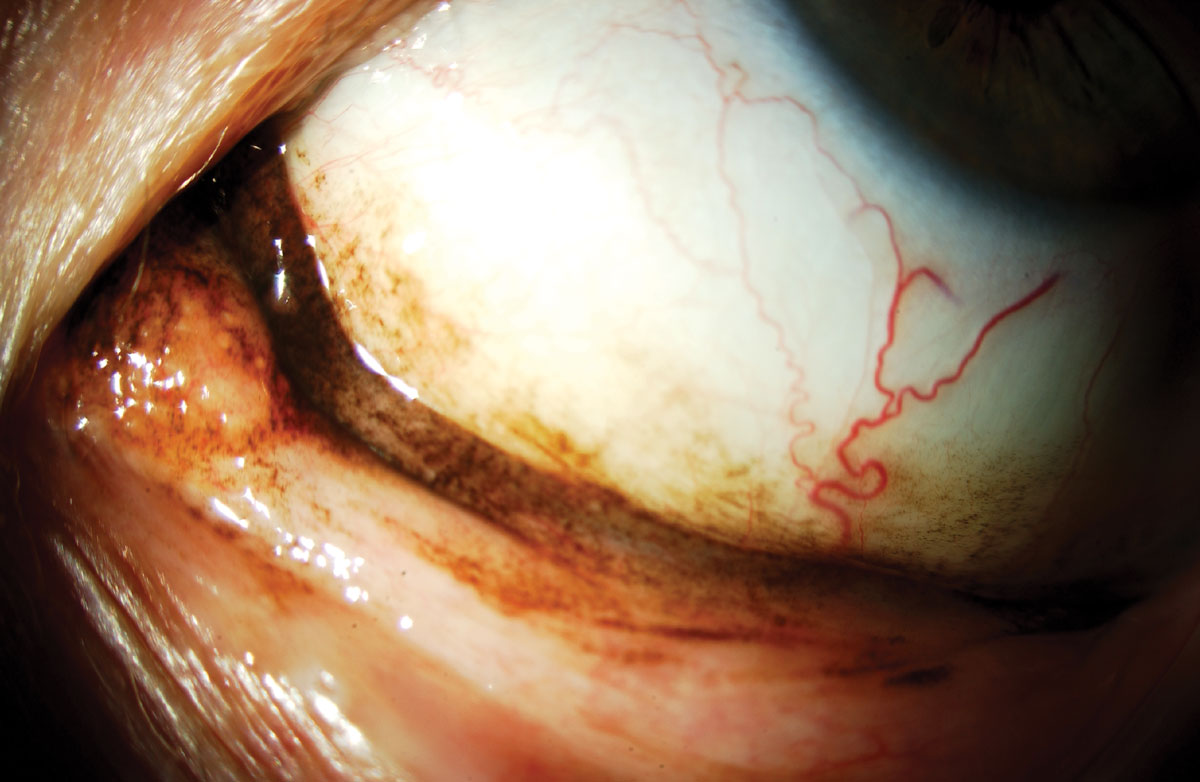 |
Fig. 3. Malignant melanoma of the conjunctiva. Click image to enlarge. |
Malignant Melanoma
One of the more serious skin cancers that can be found around the eyes is a malignant melanoma, which is cancer from the malignant transformation of melanocytes in the skin and can occur anywhere on the body, including the conjunctiva, eyelids, as well as on the iris or choroid (Figure 3).10,11 The stage when diagnosed dictates the prognosis. For example, stage I melanoma has a five-year survival rate of about 97%, whereas stage IV has a 10% survival rate.10
The five key signs are known by the mnemonic of ABCDE:
• A—Asymmetry. If you could fold the lesion in half, it wouldn’t line up.
• B—Irregular borders.
• C—Color. Portions of the lesion are brown and others are black.
• D—Diameter. If it’s over 6mm in size, it is highly suspicious for a malignant melanoma.
• E—Evolving. Lesion is growing, spreading or changing in some fashion.
Although melanomas are the most common intraocular tumor, they are rare on the ocular surface. Metastasis of ocular melanomas are typically through the lymph nodes and the most common site is the liver.12
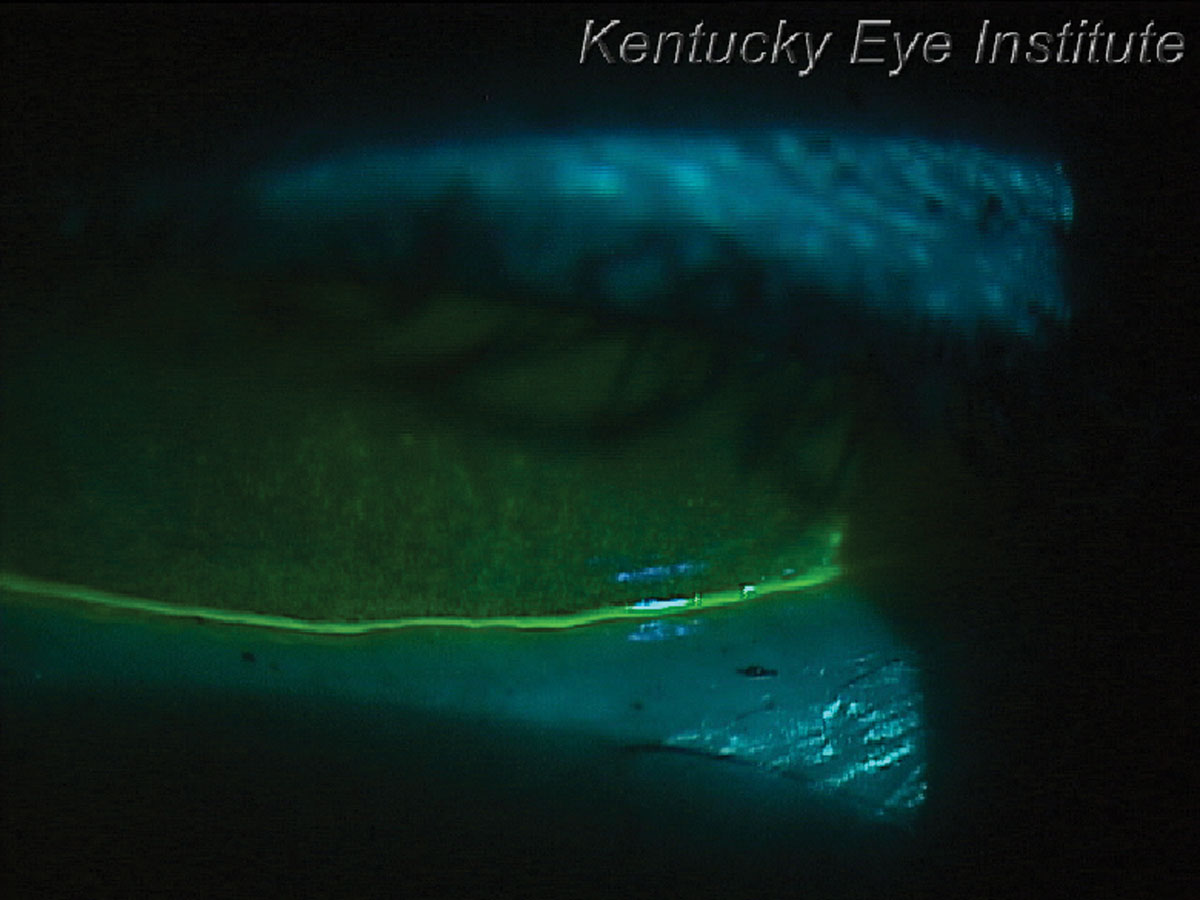 |
Fig. 4. Thin tear meniscus height and significant corneal staining in a patient with Sjögren’s syndrome keratoconjunctivitis sicca. Click image to enlarge. |
Non-Hodgkin Lymphoma
Non-Hodgkin lymphoma is the most common hematological malignancy in the world, accounting for nearly 3% of cancer diagnoses and deaths.13 Sjögren’s syndrome is most often diagnosed in patients with dry eye symptoms and keratoconjunctivitis sicca (Figures 4 and 5).14 Patients tend to show patchy corneal staining with a thin tear meniscus height. They often complain of concurrent xerostomia or dry mouth and may carry a water bottle with them to the eye exam. This condition is commonly diagnosed by primary eyecare providers as dry eye patients typically see them first.14Once the diagnosis is made, either by serology or sometimes a lip biopsy, patients receive aggressive dry eye therapy. Sjögren’s syndrome patients should also be educated about the 16 times greater risk of developing non-Hodgkin lymphoma, but also have higher risks of other forms of cancer.15 Patients with primary Sjögren’s syndrome have a 53% higher likelihood of developing malignancies.16 It is important to educate them about the symptoms of non-Hodgkin lymphoma including night sweats, unexplained weight loss, chronic fatigue and swollen lymph nodes in the neck, axillary region or groin. Medications that can be considered include monoclonal antibodies such as rituximab that affects B-cells and targets both Sjögren’s syndrome and non-Hodgkin lymphoma.17
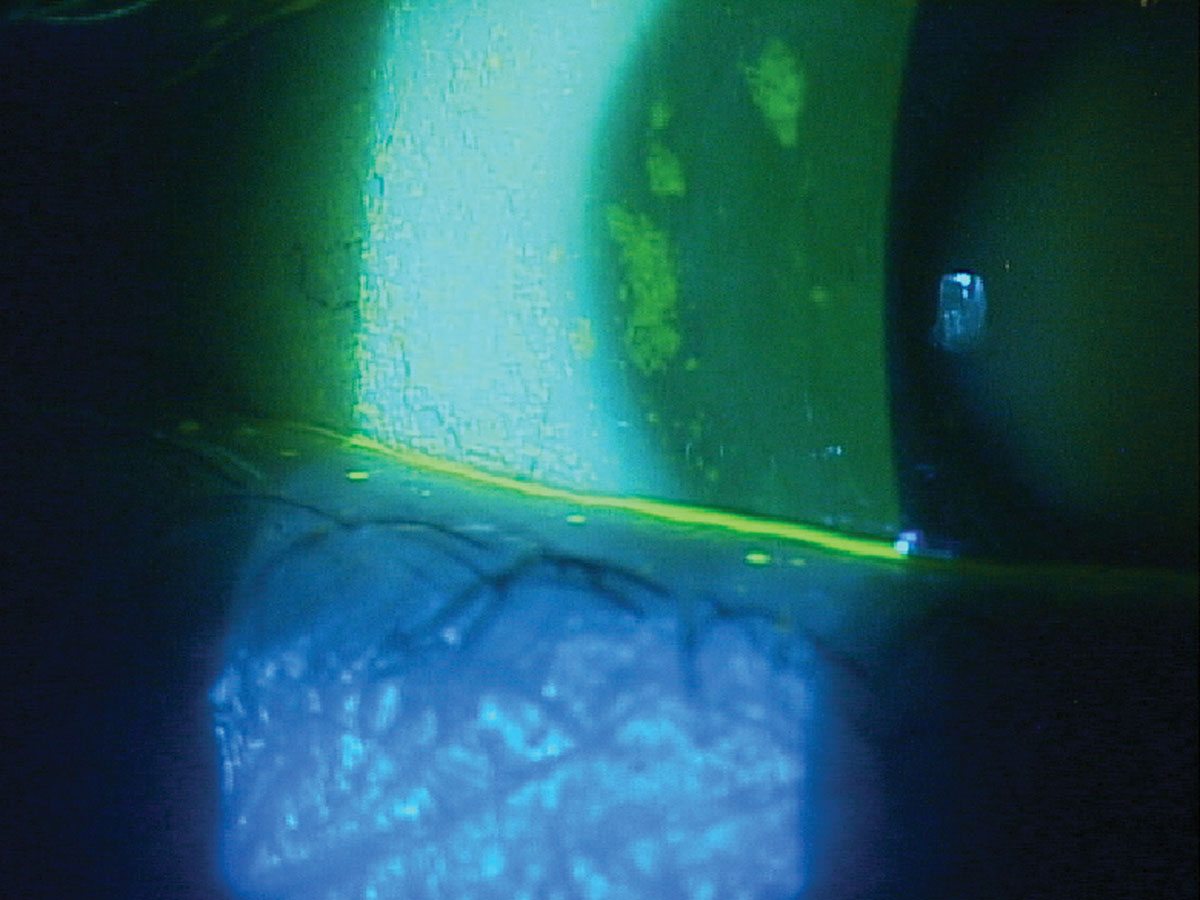 |
Fig. 5. Patchy staining and minimal tear meniscus height in a patient with Sjögren’s syndrome keratoconjunctivitis sicca. Click image to enlarge. |
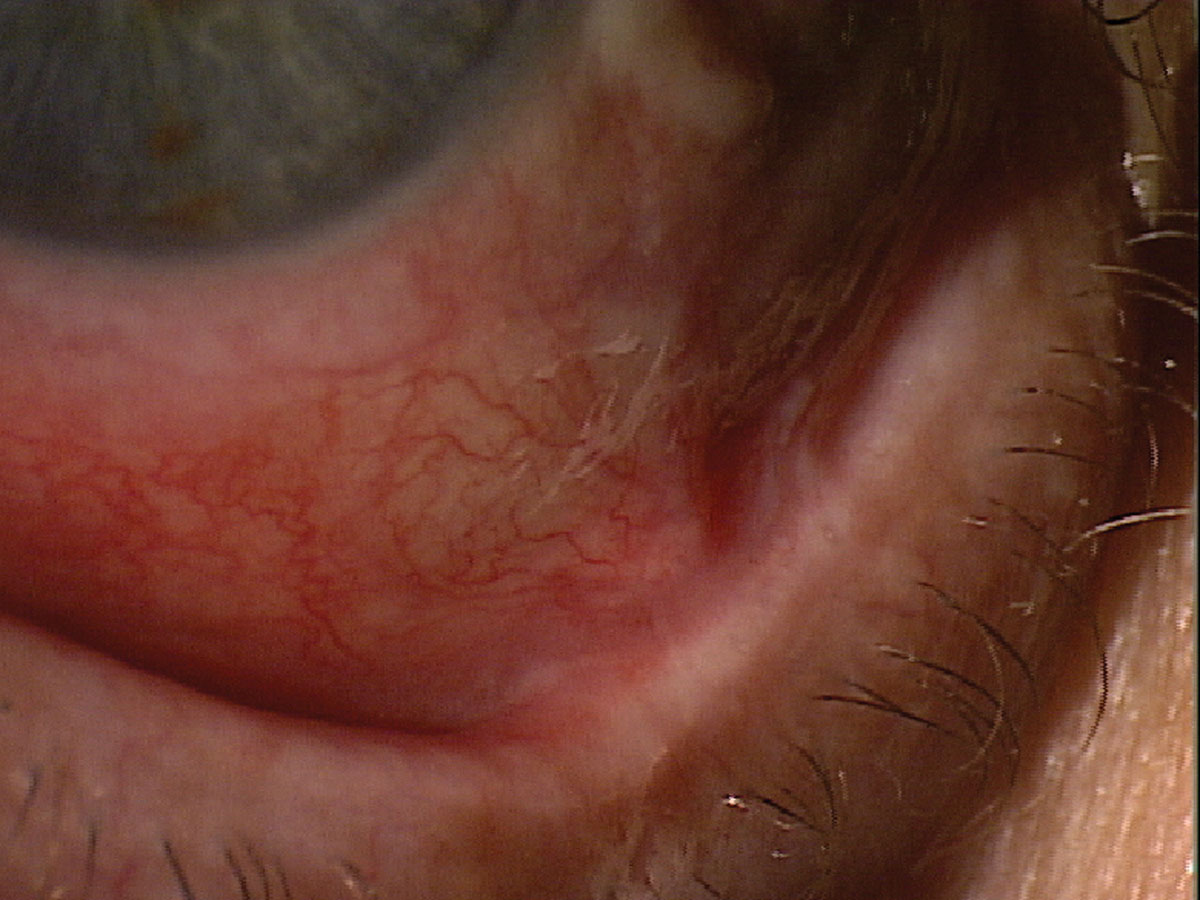 |
|
Fig. 6. This patient presented for a dry eye evaluation. The very shortened fornix suggests ocular cicatricial pemphigoid. Click image to enlarge. |
Ocular Cicatricial Pemphigoid
As the disease progresses, photophobia, foreign body sensation and pain become more common. By examining the lower fornix to see if it is shortened, one can make a positive initial diagnosis of ocular cicatricial pemphigoid (Figures 6 and 7). Patients are initially treated with topical steroids but a systemic diagnosis needs to be made. This is accomplished via a biopsy identifying the presence of IgG, IgA and/or complement components C3 or C4. Patients with ocular cicatricial pemphigoid may be treated systemically with oral dapsone or systemic immunomodulatory therapy like methotrexate, and oral steroids typically prescribed by a rheumatologist.20 These medications often begin with a lower dose and increase over time based on tolerability. Ocular management involves routinely epilating lashes, using topical corticosteroids and immunomodulators. Another successful option are adrenocorticotropic hormone analogue injections (Acthar) bi-weekly, which are often prescribed by eyecare providers.21
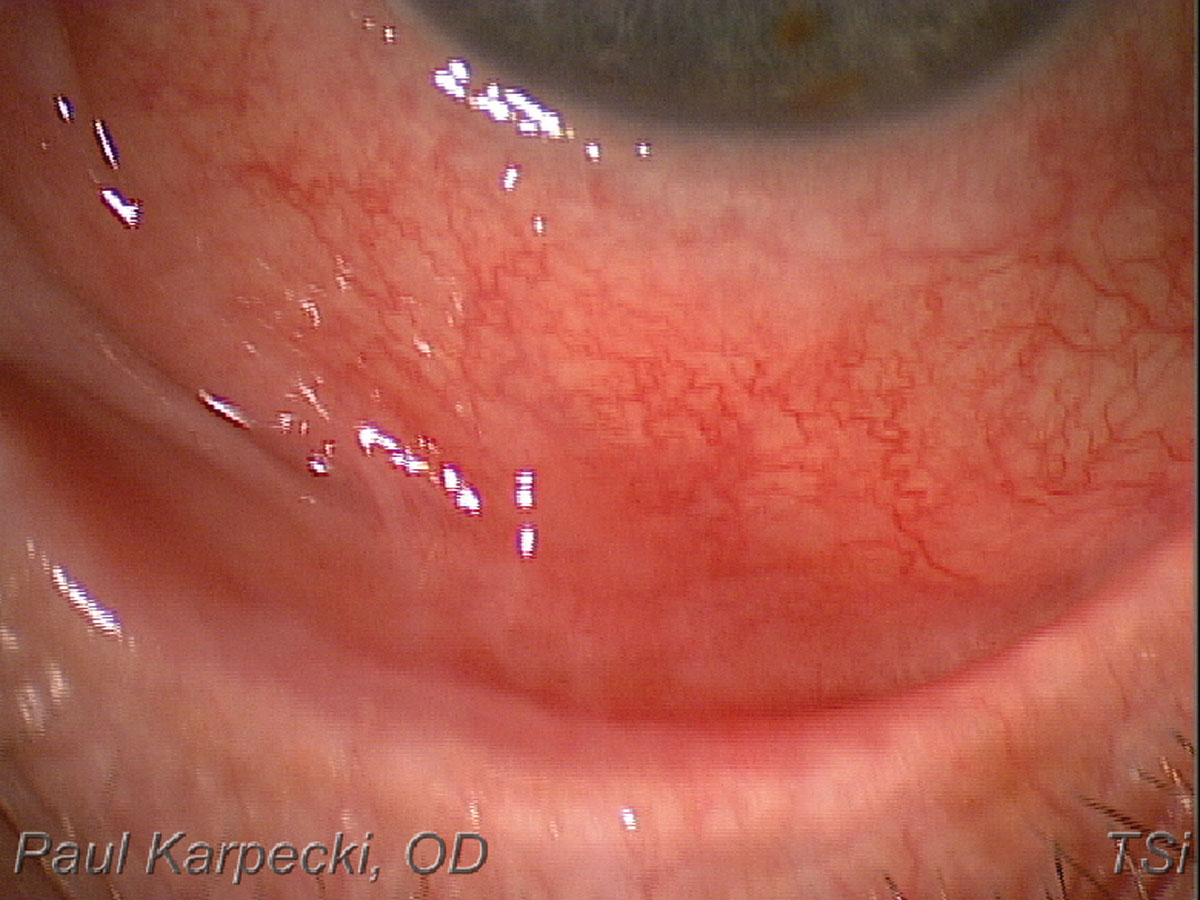 |
Fig. 7. A shortened inferior fornix, indicative of ocular cicatricial pemphigoid. Click image to enlarge. |
Ocular Graft-vs Host Disease
This is a common complication following a hematopoietic stem cell (bone marrow) transplant.22 Although ocular GVHD is not one of the three most common presentations (skin, mouth and GI tract), some form of GVHD occurs in approximately 40% to 60% of patients.23 Ocular GVHD presents as keratoconjunctivitis sicca manifesting moderate to severe ocular surface staining, then eventual scarring of the lid margins and conjunctiva (Figure 8). Most patients are treated with autologous serum drops, topical immunomodulators, placenta-based drops (RegenerEyes), amniotic membranes (e.g. Prokera or Atlas Apollo), corticosteroids and/or scleral lenses. Scleral lenses have been shown to be effective in mitigating complications and improving the ocular surface of ocular GVHD patients.24
The condition can vary from mild to life-threatening. The hope is that the GVHD will dissipate as the body begins to make its own lymphocytes from the donor cells. Chronic GVHD is the most common cause of death in patients receiving a bone-marrow transplant. Even with newer medications, the 10-year survival rate—post diagnosis—is about 42%.25 Risks increase if there is a slight mismatch, such as a donor that is not a family member. New therapies such as autologous mesenchymal stromal stem cells may improve survival rates and morbidity.26 It is important to include a history of a bone marrow or hematopoietic stem cell transplant in your OSD work-up.
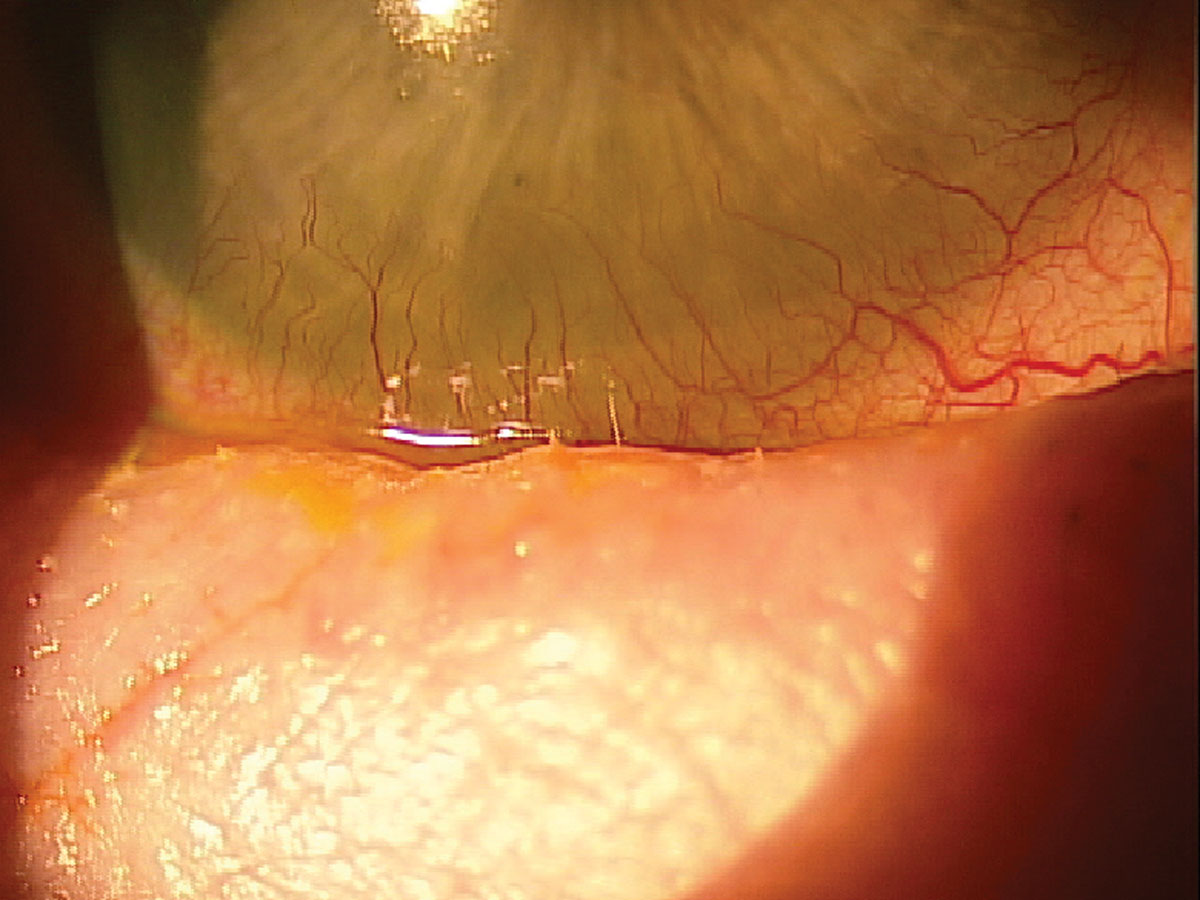 |
Fig. 8. A patient with GVHD experiencing trichiasis and severe inflammation. Click image to enlarge. |
OSDs are not all benign and need to be considered during an examination, especially in cases not responding to treatment. Examine the eyelids and adnexa thoroughly, including the areas around the nose, understand links between severe forms of dry eye and cancers and identify rare but significant presentations of keratoconjunctivitis sicca. These steps are imperative to sound clinical practice and saving patients well-being, and sometimes their lives.
1. Shields JA, Demirci H, Marr BP, et al. Sebaceous carcinoma of the eyelids: personal experience with 60 cases. Ophthalmology. 2004;111(12):2151-7. 2. Owen JL, Kibbi N, Worley B, et al. Sebaceous carcinoma: evidence-based clinical practice guidelines. Lancet Oncol. 2019;20(12):e699-714. 3. Sargen MR, Cahoon EK, Lynch FC, et al. Sebaceous carcinoma incidence and survival among solid organ transplant recipients in the United States, 1987-2017 JAMA Dermatol. 2020;156(12):1307-14. 4. Sargen MR, Starrett GJ, Engels EA, et al. Sebaceous carcinoma epidemiology and genetics: emerging concepts and clinical implications for screening, prevention and treatment. Clin Cancer Res. 2021;27(2):389-93. 5. Vissers G, Corthouts J, Van Haverbeke C, et al. Misdiagnosis of sebaceous carcinoma. Acta Chir Belg. 2022;122(2):127-32. 6. Kim DP, Kus KJB, Ruiz E. Basal cell carcinoma review. Hematol Oncol Clin North Am. 2019;33(1):13-24. 7. Teng Y, Yu Y, Li S et al. Ultraviolet radiation and basal cell carcinoma: an environmental perspective. Front Public Health. 2021:9:666528. 8. Kuflik AS, Janniger CK. Basal cell carcinoma. Am Fam Physician.1993;48(7):1273-6. 9. Petreanu CA, Șerban ED, Constantin MM, et al. Basal cell carcinoma—not always the ‘good guy’: case report of a life-threatening basosquamous carcinoma and review of the literature. Exp Ther Med. 2021;22(4):1158. 10. Heistein JB, Acharya U, Kumar S. Malignant melanoma. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. January 2023. Updated May 2023. 11. Oglesby CD. Malignant melanoma. Am J Ophthalmol. 1964:58:136-7. 12. Balasubramanya R, Selvarajan SK, Cox M, et al. Imaging of ocular melanoma metastasis. Br J Radiol. 2016;89(1065):20160092. 13. Thandra KC, Barsouk A, Saginala K, et al. Epidemiology of non-Hodgkin’s lymphoma. Med Sci (Basel). 2021;9(1):5. 14. Bjordal O, Norheim KB, Rødahl E, et al. Primary Sjögren’s syndrome and the eye. Surv Ophthalmol. 2020;65(2):119-32. 15. Solans-Laqué R, López-Hernandez A, Bosch-Gil JA, et al. Risk, predictors and clinical characteristics of lymphoma development in primary Sjögren’s syndrome. Semin Arthritis Rheum. 2011;41(3):415-23. 16. Zhong H, Liu S, Wang Y, et al. Primary Sjögren’s syndrome is associated with increased risk of malignancies besides lymphoma: a systematic review and meta-analysis. 2022;21(5):103084. 17. Tarella C, Gueli A, Ruella M, et al. Lymphocyte transformation and autoimmune disorders. Autoimmun Rev. 2013;12(8)802-13. 18. Schonberg S, Stokkermans TJ. Ocular Pemphigoid. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. July 31, 2023. 19. Besagar S, London AO, Jairam MP, et al. Chronic early-stage ocular cicatricial pemphigoid. Ophthalmic Plast Reconstr Surg. 2021;37(6):e209-13. 20. Chang JH, McCluskey PJ. Ocular cicatricial pemphigoid: manifestations and management. Curr Allergy Asthma Rep. 2005;5(4):333-8. 21. Sharon Y, Chu DS. Adrenocorticotropic hormone analogue as novel treatment regimen in ocular cicatricial pemphigoid. Am J Ophthalmol Case Rep. 2018:10:264-7. 22. Ramachandran V, Kolli SS, Strowd LC. Review of graft-versus-host disease. Dermatol Clin. 2019;37(4):569-82. 23. Choi SW, Reddy P. Current and emerging strategies for the prevention of graft-versus-host disease. Nat Rev Clin Oncol. 2014;11(9):536-47. 24. Magro L, Gauthier L, Richet M, et al. Scleral lenses for severe chronic GvHD-related keratoconjunctivitis sicca: a retrospective study by the SFGM-TC bone marrow transplant. 2017;52(6):878-82. 25. Tan Y, Shan L, Zhao L, et al. Long-term follow-up of donor-derived CD7 CAR T-cell therapy in patients with T-cell acute lymphoblastic leukemia. J Hematol Oncol. 2023;16(1):34. 26. Stenger E, Giver CR, Langston A, et al. Safety of autologous freshly expanded mesenchymal stromal cells for the treatment of graft-versus-host disease. Front Immunol. 2022:13:959658. |

