Annual Cornea ReportCheck out these articles: Skip the Scalpel? A Medical Approach to Endo Recovery |
The corneoscleral limbus is a unique and critical ocular structure responsible for barrier protection, corneal regeneration and wound repair. These functions are pivotal in maintaining corneal transparency and ocular surface integrity. In addition, the limbus contains vascular and lymphatic vessels that serve to provide nutrients and cytokines to the surrounding structures. This makes the peripheral cornea more susceptible to immune-mediated inflammation and ensuing structural changes.
The corneal epithelium is in a constant state of remodeling. The layer of surface cells, wing cells and basal cells. Surface cells are made up of non-keratinized, stratified squamous cells.1 The conjunctival epithelium, in contrast, is made up of non-keratinized, stratified columnar cells with mucin-containing goblet cells.2 As surface corneal epithelial cells are shed, the basal lamina encourages new cell proliferation. Only cells in contact with the basal layer of the epithelium can divide.3 The corneal epithelium is thought to repair itself in a centripetal fashion.4 Differentiation and replenishment of the epithelium from basal cells to surface cells takes approximately seven to 14 days to complete.5
The limbus marks the junction where the corneal epithelium meets the conjunctival epithelium. In the basal lamina at the limbus, stem cells can be found in the palisades of Vogt.6-8 These are critical for cell proliferation and migration following an insult to the corneal epithelium. Limbal stem cells also provide a barrier to conjunctival epithelial cells, keeping them from migrating onto the cornea. When limbal stem cells are damaged, the cornea can become “conjunctivalized.” Conjunctivalization is evident through neovascularization, the presence of goblet cells and an unstable epithelium.9 This transformation of cell tissue can lead to chronic inflammation, neovascularization, persistent epithelial defects and possible opacification.
The cornea has angiogenic and immune privilege, both of which are necessary to retain transparency. The process of maintaining corneal avascularity is ongoing. When the cornea is injured, anti-angiogenic factors are upregulated, while pro-angiogenic factors are downregulated. This equilibrium ensures no corneal neovascularization ensues. When the limbal tissue is compromised, balance is disrupted and angiogenic factors prevail, leading to new blood vessel growth.10-12
This article highlights several limbal diseases, to which we should pay particularly close attention.
 |
|
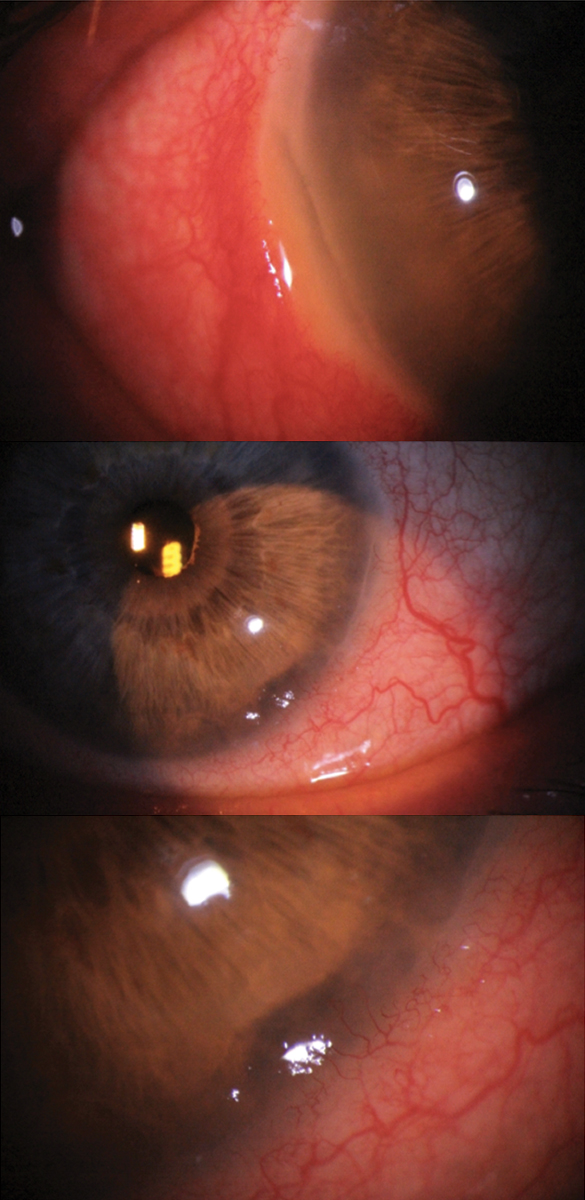
Bilateral Mooren’s ulcer in a 72-year-old white male. The right eye shows limbal melt from 7 to 9 o’clock, while the left eye shows limbal melt from 4 to 5 o’clock. Although scleritis was present in the right eye, this patient tested negative for collagen vascular disease. Due to the bilateral nature and absence of rheumatologic disease, Mooren’s was the working diagnosis rather than peripheral ulcerative keratitis, although both were differentials. Click image to enlarge. |
Deficiency Processes
Disease or trauma can bring on a state of limbal stem cell deficiency (LSCD). This condition results from dysfunction, damage or destruction of limbal stem cells. Destruction can be caused by chemical or thermal burns, Stevens-Johnson syndrome, multiple ocular surgeries, contact lens (CL) wear or severe microbial keratitis.13 Gradual loss of stem cell function may be due to a variety of genetic or acquired conditions. Genetic causes of LSCD include aniridia, congenital erythrokeratodermia and multiple endocrine deficiency.8,13 Acquired gradual loss can be stimulated by neurotrophic keratitis, vernal conjunctivitis or peripheral inflammatory keratitis, to name a few.13,14
When corneal conjunctivalization and persistent epithelial defects are present, consider LSCD. Fluorescein pooling is often evident in areas of conjunctivalization due to thin, irregular tissue with a loss of tight junctions.15 This staining can take on a whorl-like pattern and be sectoral or more widespread depending on the severity of LSCD.16 Fibrovascular pannus, scarring and calcification are also often observed. LSCD patients are highly susceptible to epithelial erosions and report chronic pain, blurred vision and photophobia.15 In advanced disease, corneal melt or perforation is possible.
Topical steroids are considered the first-line therapy in early stages of LSCD. Additionally, frequent administration of preservative-free artificial tears is recommended to treat underlying ocular surface disease. Restasis (cyclosporine, Allergan) or Xiidra (lifitegrast, Novartis) can help decrease inflammation. A bandage lens, scleral lens or amniotic membrane can promote epithelial healing. In cases where some limbal stem cells are still functional, debride the abnormal epithelium. This allows the remaining functional stem cells to repopulate the surface with normal corneal epithelium.
In extensive or complete LSCD, limbal stem cell transplantation (LSCT) is often required, as these patients do not typically respond well to traditional keratoplasty.9,13 To improve surgical outcomes, control inflammation prior to transplantation. In unilateral LSCD, autologous limbal tissue can be transplanted from the fellow eye. There is no risk of rejection in an autologous transplant, leading to a higher success rate. Bilateral LSCD, which most commonly occurs secondary to burns, requires allogenic transplantation or cultivated oral mucosal transplantation. Amniotic membranes are used to transplant tissue onto the host cornea.16 The risk of rejection in allogenic LSCT is a significant concern, necessitating the use of HLA typing and long-term immunosuppression.15,16
Common adverse effects of LSCT include recurrent or persistent epithelial erosions and increased intraocular pressure. Administer the same therapies used in early disease post-transplantation to reduce inflammation, suppress the immune system and optimize the ocular surface.
 |
|
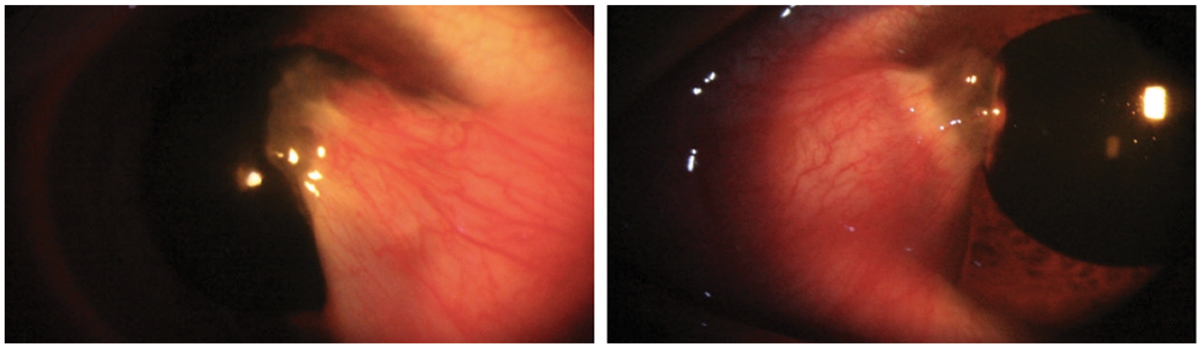
Bilateral thick pterygia in a 33-year-old Hispanic male. Pterygium excision with a conjunctival allograft was recommended in the right eye secondary to the degree of encroachment on the line of sight and significant irregular astigmatism induced by the ptergium. Click image to enlarge. |
Mechanical Processes
The ocular surface is continuously buffeted by internal and external forces and can succumb when overtaxed.
CL-mediated issues. Lens wear can lead to limbal dysfunction via mechanical wear, decreased tear exchange and corneal hypoxia. Additionally, injury is possible with improper lens insertion or removal techniques. These factors open the door to possible microbial infection and progressive compromise to the limbal tissue. CL wear and care accounts for 15% of all LSCD cases.17
Monitor the superior cornea carefully for staining due to increased friction between the superior lid and lens.16 Also monitor the periphery for neovascularization, which indicates hypoxia. Ask the patient heed their replacement schedule and consider reduced wear time and/or daily disposables to keep the risk of CL-related limbal complications low. In the case of lens-associated LSCD, discontinue lens wear indefinitely.
Superior limbic keratoconjunctivitis (SLK). This is thought to be caused by a poor interaction between the superior eyelid and the superior bulbar conjunctiva.18-20 It may also be influenced by a localized tear deficiency.20 SLK is much more common in females and may be accompanied by concurrent thyroid disease in up to 50% of cases.21
SLK is marked by superior bulbar injection, especially near the limbus, with thickening or redundancy of the conjunctival tissue. Fine papillae can be observed on the superior tarsus, and punctate erosions can be seen on the superior cornea, limbus and bulbar conjunctiva. Superior filaments often develop, and dry eye disease is a common comorbidity. Patients with SLK often present with adjunctive lid findings, such as superior lid swelling, blepharospasm and inflammatory ptosis.21
Initial therapy may include frequent lubrication, punctal occlusion and a therapeutic soft CL. Also consider autologous serum, cyclosporine A and topical mast cell stabilizers. SLK responds poorly to topical steroids.18 More aggressive treatments include 0.5% to 1% silver nitrate application to the conjunctiva following a topical anesthetic, which chemically debrides the inflamed conjunctival tissue. Take extreme caution to avoid burning the cornea. Additionally, conjunctival resection or thermal cautery of the superior bulbar conjunctiva can improve the conjunctival interface.21
Dellen. This is a painless area of thinning caused by ocular surface dryness. It often develops adjacent to elevated areas. It can also occur near conjunctival chemosis, episcleritis, pingueculae or pterygia. The presenting location is often temporal near the limbus.
Dellen commonly present following cataract surgery, strabismic surgery or glaucoma-filtering surgery.22 Patients may report mild discomfort or foreign body sensation. Look for a saucer-like depression at the slit lamp. Corneal dellen represent thinning of the epithelium, Bowman’s layer and the anterior stroma. They typically resolve within a few days but in some cases may last weeks.23 Treatment consists of frequent lubrication, patching if necessary and possibly a prophylactic antibiotic ointment. In more resistant cases, reducing the adjacent elevation may be required.
 |
|
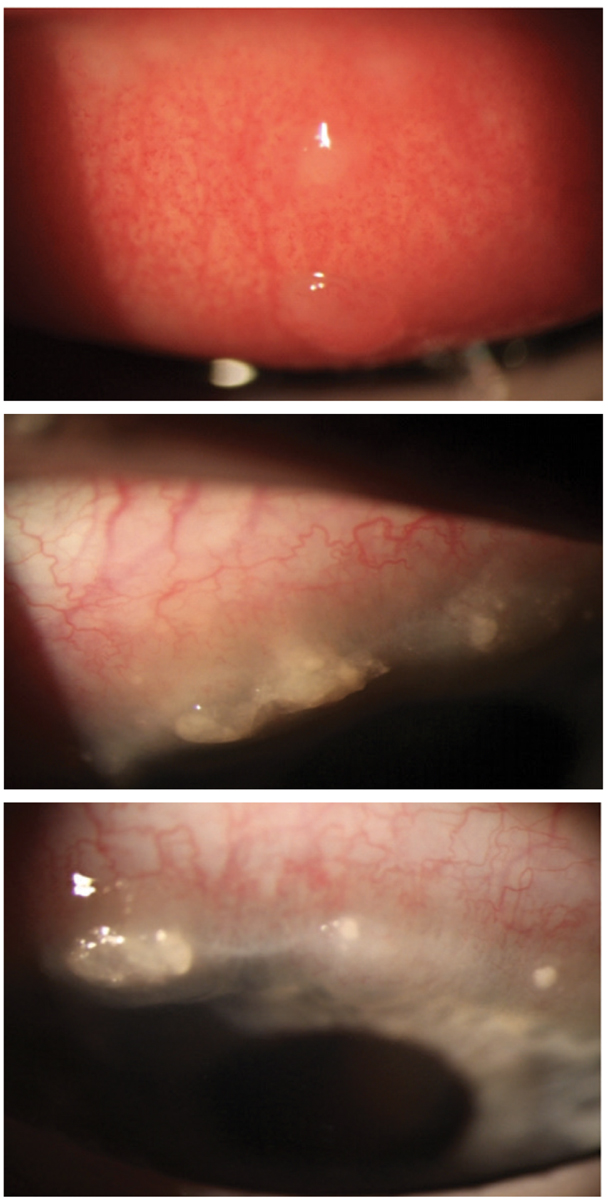
VKC with two giant cobblestone papillae and extensive Horner-Trantas dots in a nine-year-old white male. The patient responded well to treatment with topical steroids, but limbal scarring did result upon resolution. Click image to enlarge. |
Immune-mediated Processes
Aberrations in the immune response can affect the limbal region.
Phlyctenulosis. Phlyctenular keratoconjunctivitis is an immune-mediated condition in which the cornea becomes sensitized to a microbial antigen during a delayed hypersensitivity reaction. With repeated exposure to this antigen, phlyctenules can develop on the corneal or conjunctival side of the limbus. Staphylococcus is the most common stimulus; however, historically, phlyctenulosis commonly occurred in response to tuberculoprotein.24 As antigens infiltrate the surface, exotoxins are released, causing the corneal epithelium to break down.25 This condition is more common in teenagers and has a higher predilection for females.24
A phlyctenule is a 1mm to 2 mm fleshy white nodule often accompanied by conjunctival injection. Symptoms include foreign body sensation, tearing, increased light sensitivity and possible itching. Phlyctenules on the cornea increase the severity of symptoms and have the ability to ulcerate, causing scarring and neovascularization. Corneal ulceration frequently results in a triangular-shaped anterior stromal scar. Phlyctenules can be recurrent and often occur at the edge of previous neovascularization sites.24
Some cases of phlyctenular keratoconjunctivitis are self-limiting, but topical steroids dosed QID are the treatment of choice. If a corneal epithelial defect is present, a broad-spectrum antibiotic is recommended prior to steroid initiation. Due to the risk of recurrence, it is critical to treat concurrent blepharitis. This can be achieved with lid hygiene, antibiotic ointment or even a course of oral doxycycline (if the patient is older than eight and not nursing or pregnant).
Differentials of phlyctenulosis include Salzmann’s nodules, Horner-Trantas dots in vernal keratoconjunctivitis, pingueculitis and nodular episcleritis. In episcleritis and pingueculitis, the nodules do not ulcerate. Corneal phlyctenules often resemble infectious ulcers, so it is important to rule out microbial keratitis. If suspicions of an infectious etiology arise, culturing should be performed.
Staph marginal keratitis. This common immune-mediated limbal disorder is considered a type III hypersensitivity reaction to resident Staphylococcus aureus, which is often present on the lids and lashes.24 It occurs peripherally due to its close proximity to limbal lymphatic vessels. Symptoms include pain, light sensitivity, foreign body sensation and conjunctival injection.
The condition is marked by one or often multiple small white subepithelial infiltrates 1mm to 2mm from the limbus, with a clear cornea in between. These infiltrates tend to be located in areas where the lid interacts with the corneal tissue. Ulceration is possible and indicated by sodium fluorescein staining over an infiltrate. Neovascularization can ensue in cases of ongoing inflammation.
It is treated similarly to phlyctenulosis, as it responds quickly to topical steroids. If an epithelial defect is present, add a broad-spectrum antibiotic. Additionally, lid hygiene and possibly topical or oral antibiotics are critical to reduce the likelihood of recurrence.
Mooren’s ulcer. This rare, painful peripheral corneal ulceration is marked by a wavy pattern. It is associated with non-perfusion of the superficial vascular plexus.26,27 Mooren ulcers are idiopathic in etiology but thought to be immune-mediated.28 There are three known forms:26
• Type 1, or a unilateral Mooren, is a painful, progressive form found in patients older than 60.
• Type 2, a bilateral, aggressive Mooren, occurs in younger patients and progresses circumferentially.
• Type 3, a bilateral, indolent Mooren, is marked by slow, progressive peripheral corneal guttering in middle-aged patients.
Behind the slit lamp, a swollen gray area of cornea that tends to furrow rapidly can be visualized.26 An epithelial defect and stromal thinning can also be observed. In Types 1 and 2, limbal inflammation is significant and marked by swelling and neovascularization. The ulceration often starts focally at the nasal or temporal limbus and spreads circumferentially and centrally.
Therapy depends on the severity and type of ulceration. Start with an aggressive topical steroid course and a prophylactic antibiotic.26,29 Use topical cyclosporine therapy QID as adjunctive therapy.30 In any form of epithelial defect, frequent lubrication is recommended to reduce eyelid friction and inflammatory cytokines.
Type 2 often requires IV immunosuppression with methylprednisolone followed by oral steroids. In any form, the goal of treatment is re-epithelialization and decreased inflammation. Shallow ulceration can be repaired with amniotic membrane transplantation, conjunctival resection or a conjunctival flap. For deeper ulcers, partial or total lamellar keratoplasty may be required.31
Vernal keratoconjunctivitis (VKC). This severe, bilateral and chronic allergic condition is most prominent in young boys living in warmer climates. Common comorbidities include asthma and allergic rhinitis.32 VKC is thought to be an IgE-mediated hypersensitivity, but IgG, basophil and cellular delayed-type hypersensitivities may also be involved.33
Patients usually report severe itching, photophobia, thick mucus discharge, tearing, burning, foreign body sensation, pain and possibly blurred vision. Symptoms tend to be most common in the spring. On slit lamp exam, giant cobblestone papillae can be observed on the superior palpebral conjunctiva. In addition, focal white infiltrates (Horner-Trantas dots) and sectoral conjunctival and episcleral hyperemia can be seen at the superior limbus. Corneal shield ulcers are also a possible, yet uncommon, clinical manifestation. They are sterile in nature and result from mechanical rubbing of cobblestone papillae on the cornea.33
First-line treatment includes topical antihistamines and mast cell stabilizers. Topical steroids can also be considered, especially in more severe cases. In addition, calcineurin inhibitors, NSAIDs and oral antihistamines can be employed. Avoid triggers such as eye rubbing, wind, heat and sunlight. Artificial tears and cool compresses can provide some supportive relief. In the case of corneal shield ulcers, recommend a broad-spectrum antibiotic QID until the epithelium heals.34
Kids often outgrow VKC with time, but it can take years to run its course. Vision loss is possible secondary to corneal neovascularization and subsequent scarring, but this outcome is rare.
Peripheral ulcerative keratitis (PUK). This is a broad diagnostic term used to describe peripheral corneal thinning caused by a variety of collagen vascular conditions. These autoimmune conditions include rheumatoid arthritis, polyarteritis nodosa, Wegener’s granulomatosis, inflammatory bowel disease and systemic lupus erythematosus, to name a few. Rheumatoid arthritis is by far the most common of these etiologies to cause PUK.
PUK is a unilateral condition consisting of an epithelial defect, crescent-shaped stromal inflammation with thinning and accompanying episcleritis or necrotising scleritis.35 In advanced disease, perforation can ensue, which is why prompt diagnosis and treatment is critical. PUK is the initial symptom of collagen vascular disease in 50% of cases.36
If PUK is observed in a patient with no known collagen vascular disease, thorough personal and family history and lab testing is recommended. A typical lab workup includes complete blood count with differentials, erythrocyte sedimentation rate, rheumatoid factor, antinuclear antibody, antineutrophil cytoplasmic antibodies, chest X-ray examination and liver enzymes. If associated collagen vascular disease is detected, direct therapy at managing the systemic condition with the help of a rheumatologist.
Topical steroids can be used in early disease, but often systemic immunosuppressive agents are required. First-line therapy involves systemic corticosteroids and often a cytotoxic agent.35 Common immunosuppressants include cyclophosphamide, methotrexate, azathioprine and oral cyclosporine.37 Biologics can also be considered. In addition, frequent lubrication with preservative-free tears and possibly a bandage CL can improve the corneal microenvironment, as there is a high rate of concurrent dry eye disease in these patients. In more severe disease, surgical intervention may be required. Surgical techniques include tissue adhesive, lamellar graft, tectonic corneal grafting and amniotic membrane transplantation.35
Neoplastic/Deposition Processes
Accumulations of tissue or other material form yet another threat to limbal health and function.
Pterygia. These are marked by fibrovascular tissue extending onto the cornea, caused primarily by UV exposure. The fibers are thought to develop from damaged fibroblasts. Pterygia destroy Bowman’s layer, and an iron line can often be observed at the leading edge, which consists of a flat gray zone.22
Pterygia are most common nasally. Symptoms include foreign body sensation, irritation and photophobia. These growths can induce irregular astigmatism, leading to a decrease in best-corrected visual acuity, especially as they encroach on the visual axis. Frequent lubrication is recommended for symptomatic pterygia, and topical NSAIDs or steroids can be used in cases of active inflammation. Perform surgical excision if the line of sight becomes threatened.
Band keratopathy, caused by deposition of calcium in Bowman’s, iis often stimulated by chronic uveitis or hypercalcemic conditions such as chronic kidney failure. Band keratopathy begins peripherally on the nasal or temporal side and is marked by a gray opacity that can become white and chalky. The edge of the opacity is often separated from the limbus by a lucent zone. This is thought to be caused by an absent Bowman’s layer or inability of limbal vessels to prevent calcium deposition.22
Band keratopathy can break through the epithelium in later stages of disease. Also in advanced presentations, calcific deposition can extend horizontally across the cornea from limbus to limbus. Additionally, hyaline material and fibrous neovascularization can surround the calcified lesion. Thankfully, these lesions progress slowly.
If the etiology is unknown, lab testing should include serum calcium, phosphorus, uric acid and renal function. Hyperparathyroid and sarcoid testing with PTH and ACE, respectively, should also be considered. Also, ask patients about vitamin and calcium supplement intake.
Band keratopathy is asymptomatic in its early stages, but patients can experience decreased acuity, foreign body sensation, tearing and light sensitivity in more advanced disease. Early stages only require management of the underlying etiology. Surgical intervention is indicated if the deposition is encroaching on the line of sight or if the deposits are causing surface discomfort. First-line surgical therapy is epithelial debridement followed by the administration of EDTA, a chelating agent. Phototherapeutic keratectomy is also effective. Amniotic membranes can be used following deposit removal to speed up the rate of healing.22
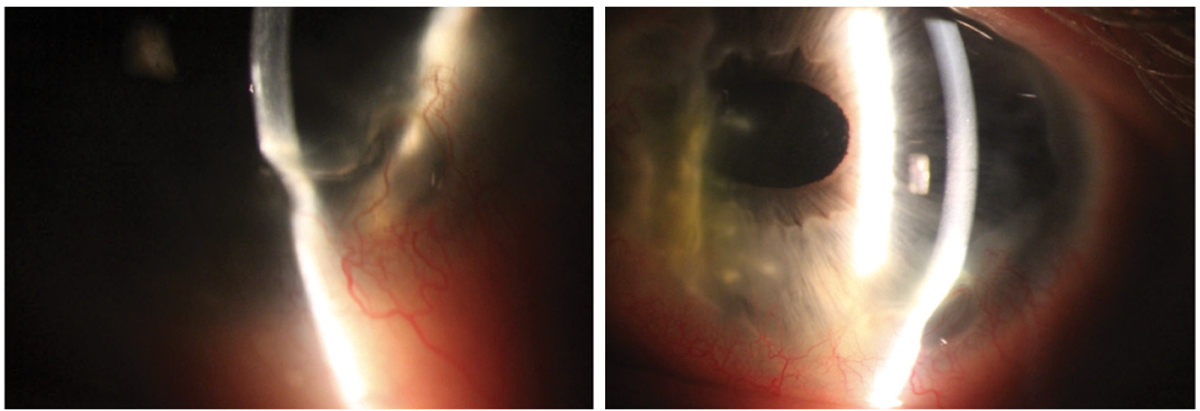 |
PUK in a 47-year-old white male. This patient had a history of prior perforation requiring a patch graft, which can be viewed on the nasal cornea. The cornea shows 70% thinning infratemporally with significant neovascularization. He required treatment with oral prednisone, slowly tapered over many months, and cyclophosphamide administered by a rheumatologist. Click image to enlarge. |
Degenerative Processes
Breakdown of corneal structures typically manifests slowly and subtly. Let’s look at two affecting the limbus.
Terrien’s marginal degeneration. This rare, asymptomatic and bilateral peripheral degeneration is idiopathic in nature and progresses slowly. It occurs most commonly in 20- to 40-year-old men and presents with a largely white and quiet eye. The degeneration begins superonasally with superficial neovascularization, punctate opacities and a gutter between the opacities and the limbus.22 The epithelium remains intact but Bowman’s and Descemet’s are disrupted. Over the course of years, the stroma continues to thin. As it does, aqueous pockets and possibly a yellow-white lipid zone can be observed.
There are two forms of this degeneration: a quiet one seen in older patients, which yields little to no symptoms, and an inflammatory form in younger patients.22 The latter is often accompanied by episodes of episcleritis or scleritis, which can be treated with steroids.38 Many cases don’t require treatment, but in the rare event of perforation, consider lamellar or eccentric grafts.22
Pellucid marginal degeneration. This painless condition with no observable inflammation is marked by bilateral inferior corneal thinning. It often causes high amounts of irregular or against-the-rule astigmatism. It tends to progress slowly and can be diagnosed with corneal topography, which commonly reveals a “kissing birds” or “crab claw” pattern. Specialty CLs such as sclerals may be indicated for optimal visual acuity.
Takeaways
The corneoscleral limbus is crucial for barrier protection, prompt corneal healing and corneal transparency maintenance. The limbus aids in fighting off infection, injury and inflammation. Maintain integrity by preserving limbal stem cells, keeping conjunctival tissue at bay and ensuring a hydrated, stable corneal surface. Restoring healthy limbal function includes adequately lubricating the ocular surface, minimizing inflammatory responses and avoiding mechanical damage. In cases of severe limbal compromise, consider amniotic membranes, grafts or LSCT to restore corneal structure and function.
Dr. Mannen practices at a private optometry and ophthalmology clinic in Northern Utah. She completed her residency at the Walla Walla Veteran Affairs Medical Center and Pacific Cataract and Laser Institute. She is a Fellow of the American Academy of Optometry. She has no financial interests to disclose.
1. Sangwan VS. Limbal stem cells in health and disease. Biosci Rep. 2001;21(4):385-405. 2. Meller D, Tseng SC. Conjunctival epithelial cell differentiation on amniotic membrane. Invest Ophthalmol Vis Sci. 1999;40(5):878-86. 3. Lavker RM, Dong G, Cheng SZ, et al. Relative proliferative rates of limbal and corneal epithelia. Implications of corneal epithelial migration, circadian rhythm, and suprabasally located DNA-synthesizing keratinocytes. Invest Ophthalmol Vis Sci. 1991;32:1864-75. 4. Thoft RA, Friend J. The X, Y, Z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci. 1983;24(10):1442-3. 5. Hanna C, Bicknell DS, O’Brien JE. Cell turnover in the adult human eye. Arch Ophthalmol. 1961;65:695-8. 6. Davenger M, Evensen A. Role of the pericorneal peripapillary structure in renewal of corneal epithelium. Nature. 1971;229(5286):560-1. 7. Schermer A, Galvin S, Sun TT. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986;103(1):49-62. 8. Kruse FE. Stem cells and corneal epithelial regeneration. Eye (Lond). 1994;8(Pt 2):170-83. 9. Dua HS, Azuara-Blanco A. Limbal stem cells of the corneal epithelium. Surv Ophthalmol. 2000;44(5):415-25. 10. Lim P, Fuchsluger TA, Jurkunas UV. Limbal stem cell deficiency and corneal neovascularization. Semin Ophthalmol. 2009;24(3):139-48. 11. Azar DT. Corneal angiogenic privilege: angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc. 2006;104:264-302. 12. Chang JH, Gabison EE, Kato T, et al. Corneal neovascularization. Curr Opin Ophthalmol. 2001;12(4):242-9. 13. Puangsricharern V, Tseng SC. Cytologic evidence of corneal diseases with limbal stem cell deficiency. Ophthalmology. 1995;102(10):1476-85. 14. Sangwan VS, Jain V, Vemuganti GK, et al. Vernal keratoconjunctivitis with limbal stem cell deficiency. Cornea. 2011;30(5):491-6. 15. Sejpal K, Bakhtiari P, Deng SX. Presentation, diagnosis and management of limbal stem cell deficiency. Middle East Afr J Ophthalmol. 2013;20(1):5-10. 16. Conto JE. A review of limbal stem cell deficiency. J Dry Eye Ocul Sur Dis. 2019;2(1):4-11. 17. Donisi PM, Rama P, Fasolo A, et al. Analysis of limbal stem cell deficiency by corneal impression cytology. Cornea. 2003;22(6):533-8. 18. Nelson JD. Superior limbic keratoconjunctivitis (SLK). Eye (Lond). 1989;3(Pt 2):180-9. 19. Cher I. Superior limbic keratoconjunctivitis: multifactorial mechanical pathogenesis. Clin Exp Ophthalmol. 2000;28:181-4. 20. Goto E, Shimmura S, Shimazaki J, et al. Treatment of superior limbic keratoconjunctivitis by application of autologous serum. Cornea. 2001;20(8):807-10. 21. Driebe WT Jr., Sakhalkar MV. Superior limbic keratoconjunctivitis. In: Krachmer JH, Mannis MJ, Holland EJ, eds. Cornea: Fundamentals, Diagnostic, Management. 3rd ed. St Louis, MO: Elsevier; 2011, 623-27. 22. Chang RI, Ching S. Corneal and conjunctival degenerations. In: Krachmer JH, Mannis MJ, Holland EJ, eds. Cornea: Fundamentals, Diagnostic, Management. 3rd ed. St Louis, MO: Elsevier; 2011, 907-15. 23. Accorinti M, Gilardi M, Giubilei M, et al. Corneal and scleral dellen after an uneventful pterygium surgery and a febrile episode. Case Rep Ophthalmol. 2014;5(1):111-5. 24. Chung G. Phlyctenular keratoconjunctivitis and marginal staphylococcal keratitis. In: Krachmer JH, Mannis MJ, Holland EJ, eds. Cornea: Fundamentals, Diagnostic, Management. 3rd ed. St Louis, MO: Elsevier; 2011, 1143-8. 25. Smolin G. Hypersensitivity reactions. In: Smolin G, ed. Ocular Immunology. 2nd ed. Boston, MA: Little, Brown; 1986. 26. Watson PG, Management of Mooren’s ulceration. Eye (Lond). 1997;11(Pt 3):349-56. 27. Garg P, Sangwan VS. Mooren’s ulcer. In: Krachmer JH, Mannis MJ, Holland EJ, eds. Cornea: Fundamentals, Diagnostic, Management. 3rd ed. St Louis, MO: Elsevier; 2011, 1149-53. 28. Kafkala C, Choi J, Zafirakis P, et al. Mooren ulcer: an immunopathologic study. Cornea. 2006;25(6):667-73. 29. Sangwan VS, Zafirakis P, Foster CS. Mooren’s ulcer: current concepts in management. Indian J Ophthalmol. 1997;45(1):7-17. 30. Tandon R, Chawla B, Verma K, et al. Outcome of treatment of Mooren ulcer with topical cyclosporine A 2%. Cornea. 2008;27(8):859-61. 31. Yang L, Xiao J, Wang J, et al. Clinical characteristics and risk factors of recurrent Mooren’s ulcer. J Ophthalmol. 2017;2017:8978527. 32. Bozkurt MK, Bozkurt B, Artac H, et al. Vernal keratoconjunctivitis—a rare but serious comorbidity of allergic rhinitis and eustachian tube dysfunction. Int J Pediatr Otorhinolaryngol. 2010;74(1):60-3. 33. Bonini S, Bonini S, Lambiase A, et al. Vernal keratoconjunctivitis revisited: a case series of 195 patients with long-term followup. Ophthalmology. 2000;107(6):1157-63. 34. Cetinkaya A, Akova YA, Dursun D, et al. Topical cyclosporine in the management of shield ulcers. Cornea. 2004;23(2):194-200. 35. Yagci A. Update on peripheral ulcerative keratitis. Clin Ophthalmol. 2012;6:747-54. 36. Tauber J, Sainz de la Maza M, Hoang-Xuan T, et al. An analysis of therapeutic decision making regarding immunosuppressive chemotherapy for peripheral ulcerative keratitis. Cornea. 1990;9(1):66-73. 37. Cao Y, Zhang W, Wu J, et al. Peripheral ulcerative keratitis associated with autoimmune disease: pathogenesis and treatment. J Ophthalmol. 2017;2017:7298026. 38. Austin P. Brown SI. Inflammatory Terrien’s marginal corneal disease. Am J Ophthalmol. 1981;92(2):189-92. |

