Polish Your Dx SkillsNo matter how long you've been in practice, it's always worthwhile to brush up on your diagnostic techniques and incorporate newer ones when you can. This month in Review of Optometry, experts review how to refine your slit-lamp skills, distinguish between optic nerve differentials, use head-mounted perimetry and pay closer attention to detail in peripheral fundus photos. Check out the other articles featured in the February 2024 issue: |
Head-mounted perimetry is a rapidly expanding new modality for testing the visual field (VF). These small, portable, video-based devices that are worn by patients are looking to update and transform this workhorse diagnostic test. The traditional bowl-based devices that have been the primary method for more than four decades have several size and ergonomic limitations that hinder their use in many practice settings and with certain patients who physically can’t place their head into the device.
A head-mounted device is light enough to wear and can be brought into the exam room, potentially allowing more patients to complete the exam. In addition, the efficiencies of a shorter test time, if realized, could be a great advantage in today’s busy practices. In many models, other diagnostic tests (e.g., color vision, contrast sensitivity) may also be added into the device as it runs on its own, freeing up time the staff or doctor may not need to spend with the patient directly.
 |
|
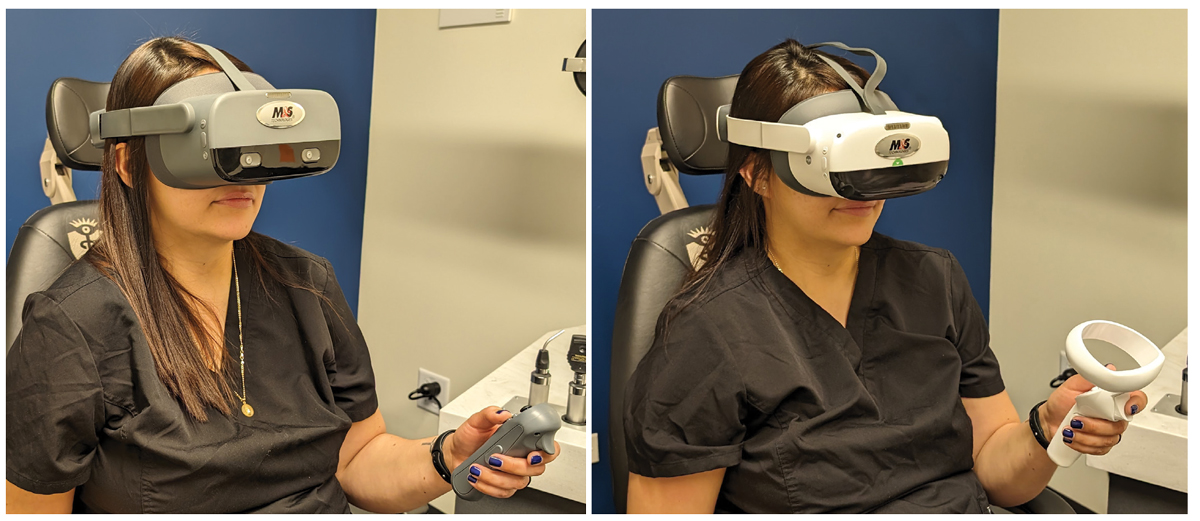
The M&S Smart System VR Headset during live testing. The latest version of the device is on the right. Click image to enlarge. |
In the last several years, perhaps a dozen different models have been introduced. The marketplace is changing so rapidly that it’s difficult to keep track of what’s in and what’s out. Perimeters are designated as a Class 1 medical devices, which pose the lowest risk, and thus are exempted from the formal premarket notification application and FDA clearance process (an OCT imaging device requires this clearance). The devices are registered by the FDA. This “low bar” of entry, along with a need for more patient- and practice-friendly visual field devices has helped spur the large number of companies (including many new start-ups) to get in the game. However, it calls into question the accuracy, sensitivity and specificity of the new head-mounted devices as there are only a small number of published articles in this area—and certainly not a refereed publication for each commercially available device.
Optometrists should be aware of certain limitations, along with the potential advantages, prior to making a purchase for their practice. Let’s take a closer look at the key issues for head-mounted perimetry devices.
Features
The current head-mounted devices (HMDs) available on the market are lightweight, ranging from 6oz to 12oz. Their ergonomic design helps improve patient comfort by not requiring them to remain in one position throughout the entire test. They also do not require the use of an eye patch as most of the virtual reality (VR) testing protocols offer simultaneous viewing while stimulating each eye individually. Allowing the patient to keep both eyes open throughout the test is typically more comfortable for the patient vs. wearing a patch. Standard Humphrey VF has a testing distance of 30cm, thus requiring trial lenses. VR allows for test stimuli to be placed at a distance of infinity, which also means the patient can wear their own habitual glasses or a custom-designed trial lens built into the headpiece, thus, eliminating a common artifact seen with standard automated perimetry.
Most of the commercially available head-mounted perimetry devices offer the common testing grid patterns (24-2, 30-2 and 10-2) while some even include kinetic, full-field 120 and screening methods for ptosis evaluation. Testing protocols use a Goldmann size lll test stimuli under mesopic testing conditions, where a white stimulus is shown against a gray or black background. The average test time slightly differs among each device while several are shorter than standard bowl perimetry.1-2
Note that there is not enough published information at this time to assess the test duration for all testing strategies on all devices. The reader is advised to investigate this carefully for each headset.
One feature that accounts for shorter test times and improved reliability of VR testing is enhanced eye tracking and monitoring capabilities. The re:Vive (Heru) platform uses an active eye tracking system via an infrared camera that will prompt the patient to regain fixation by wiggling the target if fixation is lost.3 Other platforms will pause the test when fixation is lost until the patient realigns to the target. Vivid Vision Perimetry (VVP), a VF platform designed to operate on commercially available VR headsets without a dedicated set of goggles, uses oculokinetic perimetry, where the target stimulus is dynamic and changes location from trial to trial, while the patient responds by moving their head to line up their fixation with the target.4 VR is ideally suited for these features, and some devices even include continuous monitoring during the test with AI support.
Advantages of Head-Mounted Perimetry1. Improved patient comfort. These devices can provide a more comfortable testing experience for patients compared with traditional perimetry machines.2. Increased accessibility. Portable head-mounted perimetry devices may offer increased accessibility, allowing for VF testing in various settings, including remote or underserved areas. 3. Real-time data and analytics. Some head-mounted perimetry devices can provide real-time data and analytics, enabling healthcare professionals to monitor and analyze visual field changes more efficiently. 4. Customized testing. Head-mounted perimetry devices may allow for more customized and targeted visual field testing, tailoring the assessment to specific patient needs or conditions. 5. Patient engagement. The use of modern technology, such as head-mounted perimetry, may enhance patient engagement in the testing process, potentially leading to more accurate results. |
Finding a way to make VF testing more interactive while allowing patient freedom to move their head, and eyes in some cases, is a clear benefit for the patient experience as well as the physician who receives more reliable and valid reports to interpret.5-6 The use of video instruction and monitoring eliminates the need for a technician to run the test and can improve overall clinical efficiency. Using this platform can open the possibility for other tests to be incorporated into the device. Many have already adopted other testing protocols, such as visual acuities, color vision, contrast sensitivity and extraocular motility testing, which can further increase productivity in the clinic.
The hardware for many of the current generation of headset perimeters is built around a popular VR gaming headset that is commercially available for modification (Pico Immersive). Despite having a common piece of integral hardware, the programming and software variances between HMDs can be significant.
 |
|
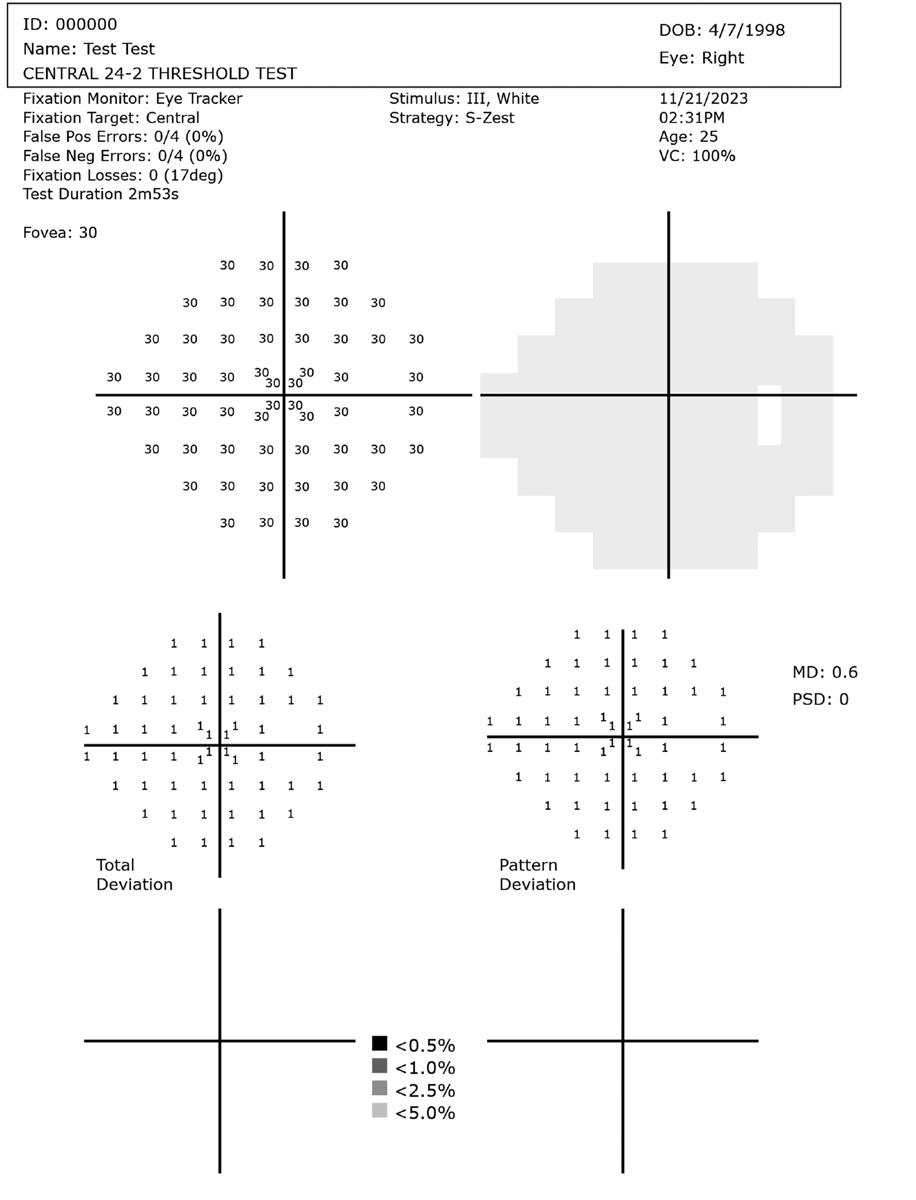
Healthy right eye visual field example for reference. These test results are from an unaffected 26-year-old using an HMD. Testing time was 2m 53s. Click image to enlarge. |
Of course, these hardware and software differences lead to questions about their similarity to the standard Humphrey Field Analyzer (HFA) bowl tests. Headset device companies have taken a variety of approaches to validating their devices. While companies may have some limited documentation of internal studies, most of this work has not been published (this is not an FDA requirement for VF testing devices).
From our review of the currently available publications, it is difficult to draw any clear conclusions regarding the sensitivity and specificity of these devices as compared with a traditional bowl perimeter.7-8 This is not to say that head-mounted perimetry units don’t work at all; they clearly do. We’ve used several of them in our clinics over the past year. The HMDs do indeed identify field defects on our glaucoma patients; however, they vary significantly in size, shape and location of the defect vs. our HFA3 device, leaving us in a quandary as to as which results are most accurate: the new shorter test that many patients seem to prefer or the gold standard device with 40 years of research and validation?
Each of the head-mounted perimetry devices offers cloud-based storage, are DICOM compatible and can be integrated into many practice EMR systems. The Radius, VF2000 and Vivid perimetry can be performed offline. This allows mobile testing opportunities such as nursing homes, hospitals and home-based perimetry. All the data is saved locally then synced with the cloud once Wi-Fi becomes available. The report generated by each device is familiar to the practitioner and features all the traditional analysis seen on HFA perimetry printouts. These include a VF greyscale plot, mean deviation, total deviation, pattern deviation and reliability indices composed of false positives, false negatives and fixation losses.
Optimal Situations
Wearable HMDs have been shown to improve patient experience and comfort while increasing clinical efficiency, but how does this fit into your practice today?5,6 We can certainly see the advantage this might offer in settings where use of standard perimetry would otherwise not be an option, such as bedridden, hospital-based or nursing home patients and those with physical limitations that prevent them from being in one position throughout the entirety of VF testing.
Practices who serve a high volume of pediatric patients could also benefit from such technology. There have been attempts to create game-based perimetry to make testing more appealing to this population. The VisuAll K VRP (Olleyes) is a pediatric video game–based static threshold perimeter that provides a format that is easy to perform and offers more reliability due to improved attention.9 A recent study was performed where a normative database was established and showed good correlation with HFA perimetry.9
 |
|
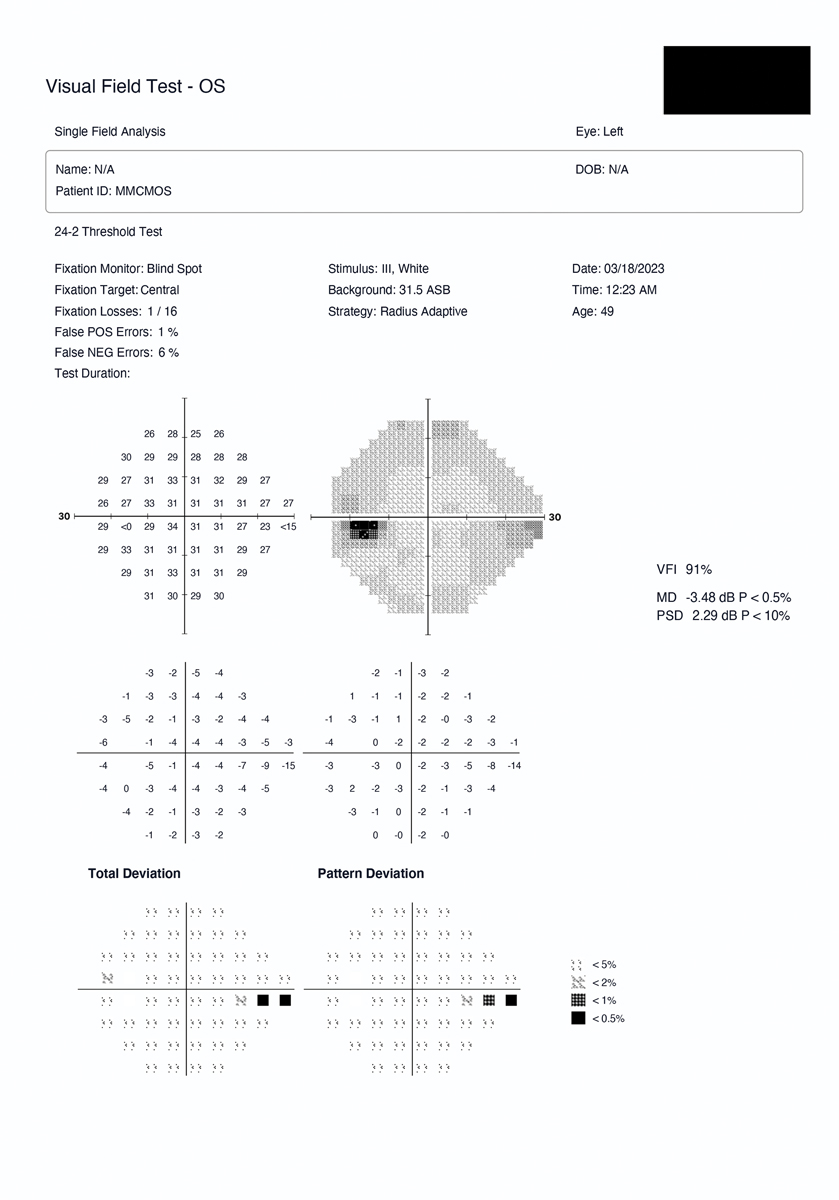
Head-mounted device example with early glaucoma defect. This was a 49-year-old glaucoma patient with an inferior nasal step in the left eye as detected by the HMD. Click image to enlarge. |
One limitation in identifying glaucoma disease and progression in those who speak non-English languages is the ability to many times conduct a reliable VF. The devices we have reviewed here all offer video instruction and continuous monitoring in multiple languages. Community health centers and practices who serve a diverse population could greatly benefit from this type of technology.
Although most studies report a high rate of patient acceptance of the HMD, sample sizes have been small and do not encompass a diverse range of the population. Wearable HMDs may be problematic for patients with head and neck injuries, those who suffer from claustrophobia or in age demographics reticent to accept advanced technology. Some patients may appreciate the progressive and innovative design, while others may be intimidated and overwhelmed, particularly the demographic where glaucoma is most prevalent. These are times when you may still want to have access to standard perimetry.
One thing the COVID-19 pandemic showed us was the vulnerability we have to clinic-based medicine. Many health specialties use telemedicine to better serve patients, especially those in rural areas or those with limited access to health care. Eyecare providers rely heavily on office-based devices which makes it difficult to offer remote care to our patients. Head-mounted perimetry could allow for home-based testing of visual fields, which might allow access for these patients, free up clinical space, as well as improve our ability to track disease progression. In the future, home-based model testing (which the patient could rent) could be performed with increased frequency.
More frequent visual fields have been reported in the literature to be superior to biannual testing in detecting rapid progression.10-11 Typically, it takes three to four fields to overcome learning curves, which could be achieved more quickly with access to increased frequency of testing.12
Artificial intelligence has been used in many areas of medicine in recent years. Use of AI to analyze structural information (such as RNFL OCT) has been studied recently to aid in detection of disease progression. This same technology could be applied to perimetry, and the current platform for VR testing may open up opportunities for future integrations with other tests. The application of AI could aid in detection and monitoring of disease and may be the future of glaucoma care.13
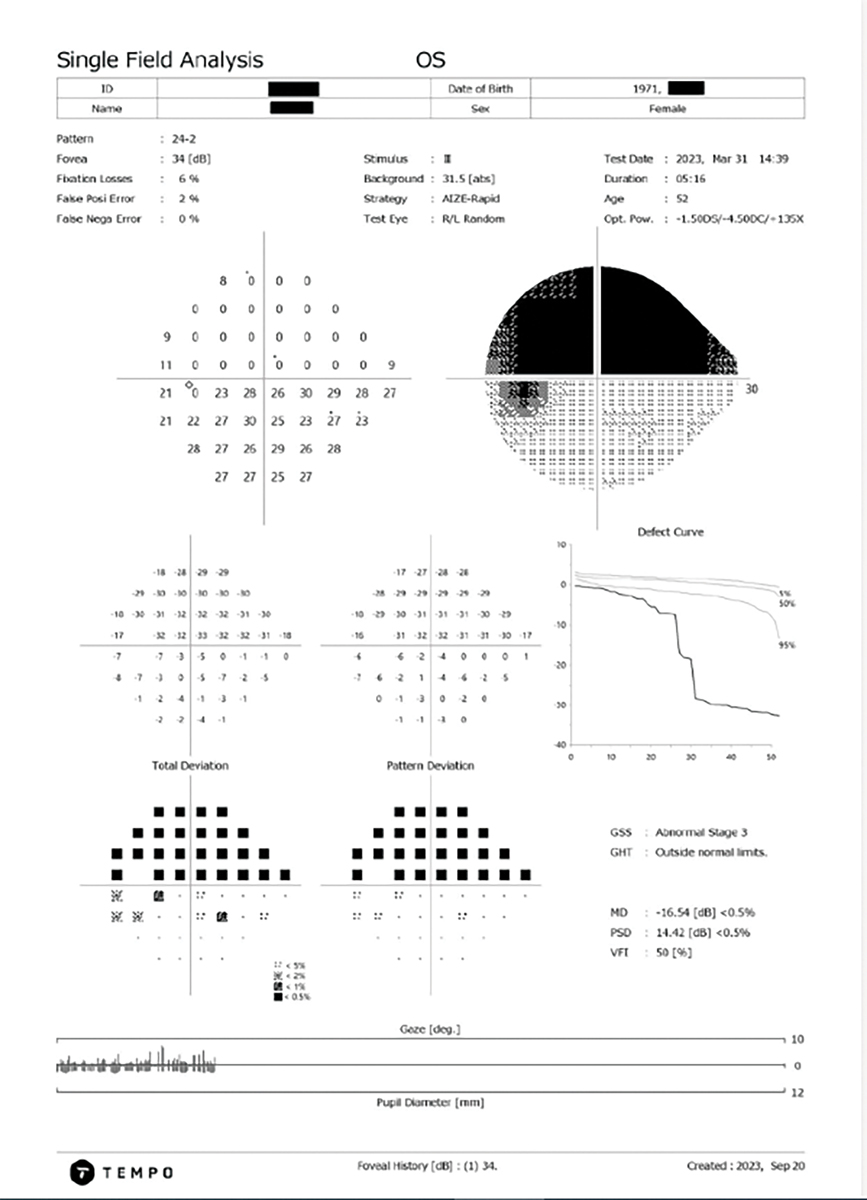
Setting a New TempoThe Tempo automated perimeter (Topcon Healthcare) is a new visual field device that bridges the gap between traditional standard automated perimetry (SAP) and wearable perimeters. An alternative to a virtual reality or head-mounted devices, this novel, non-bowl-based device maintains the technical capabilities of traditional perimetry devices. It has the advantages of a modest tabletop instrument to ensure easy positioning of a patient’s head during testing while allowing visual field tests to be conducted in ambient room lighting, eliminating the need for a dedicated dark room.
The Tempo perimeter uses AIZE, or Ambient Interactive ZEST (CREWT Medical Systems), a strategy that determines the sensitivity thresholds across the retina by using a basic algorithm of ZEST through interaction with immediate surrounding test points. As a result, it offers test performance equal to conventional standard automated perimetry while shortening measurement time.1,15-17 This perimeter takes advantage of a binocular simultaneous strategy in which stimuli are randomly presented to one eye at a time under binocular viewing conditions. This approach not only removes the risk of Ganzfeld blankout, but it may also cut inter-eye setup and improve fixational stability. The theory that a binocular simultaneous strategy in visual field testing can reduce test duration corresponds to that of the Tempo reducing measurement time by 39% in comparison to the HFA SITA-Fast.17-19 Most perimetric devices today, including the Tempo and the HFA, use a standard background luminance of 10cd/m2 (31.5 apostilbs) and a white-on-white setup of stimulus and background. This background luminance provides a low photopic adaptation that resides on the linear aspect of the Weber function; therefore, changes in pupil size or ocular media would not impact the patient’s ability to detect the stimuli. Lower background luminance can produce an uneven adaptation state from one location to another in the visual field and even require too much dark adaptation time to be useful in clinical practice. |
Limitations
Some practical limitations of VR perimetry include: (1) questions of accuracy and reproducibility as compared with established testing standards, (2) limited field of view: often only central 30° can be tested, (3) adaptation and learning effects: not all patients will perform well with a HMD, and many of our older patients get disorientated with the device on their head.
Regarding the current hardware and software limitations, there are at least three notable ones to be aware of. First, current HMDs do not always have the wide “dynamic range” that will allow for the presentation of a very dim stimulus (e.g., >35dB) nor the capability to present a very bright stimulus (<15dB). Thus, there are device limitations for fully assessing patients with deep, dense and severe field defects, such as found in moderate to severe stage glaucoma.
A second general technical difference between the HMDs and traditional devices is that the background illumination in the headset is somewhat brighter. While most perimeters have standardized background illumination to the photopic range (HFA is 10cd/m2)the HMDs have a typically have a background illumination in a dimmer mesopic range (0.01cd/m2 to 3cd/m2).14
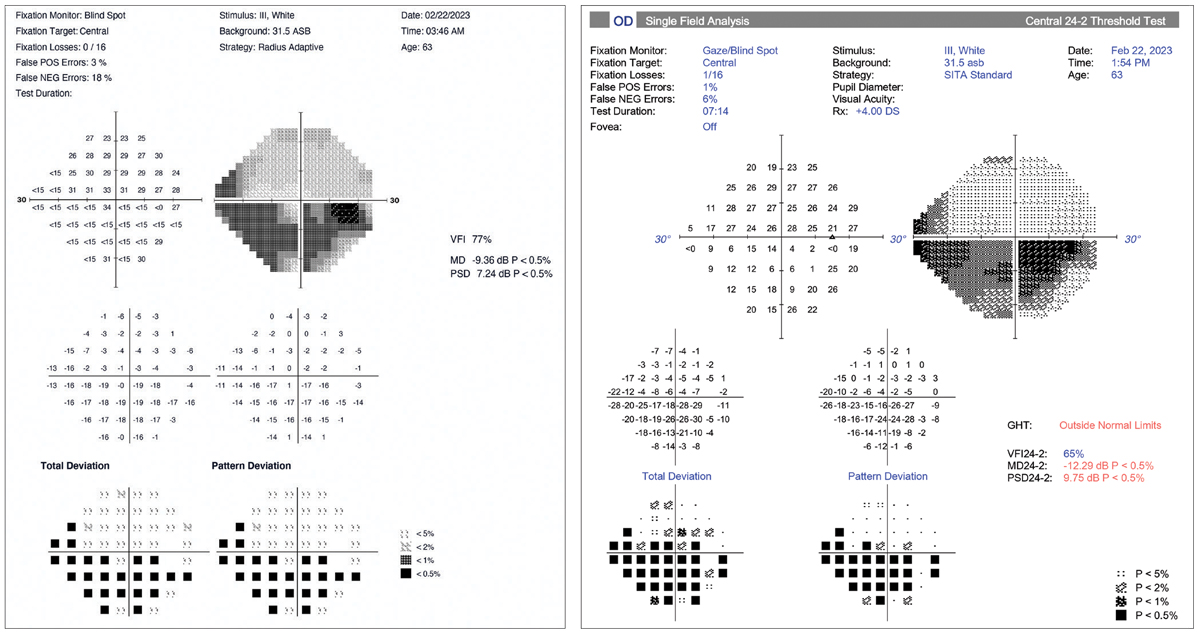 |
|
HMD (left) and HFA (right) examples of a glaucoma patient with a severe defect in the left eye. In this case, there is good general agreement between the two devices. However, there is a 3dB difference between the mean deviation values (HMD=-9.36; HFA=-12.29). Different testing strategies account likely account for this. Click image to enlarge. |
A third area of concern is the inconsistent implementation of progression analysis. This is used to track mean deviation (MD) and other parameters over a series of tests. Regression lines are calculated to identify a negative slope, which is a potential indicator for disease progression. While some of the currently available HMDs have some form of progression analysis, not all do. Readers are again reminded to evaluate each device carefully to assure that it will meet their practice needs. Clinical studies with published results are the best way to determine the full impact of these feature differences between HMDs and traditional perimeters.
Which leads to another point of concern, how does a practitioner “change over” their practice from a bowl device to an HMD? The answer is not straightforward and will vary across practice settings. Certainly it’s recognized that switching to a new diagnostic device that has different hardware and software test results between the old and new device may not be comparable and potentially could lead to an error in patient management. Generally speaking, obtaining a new baseline of HMD VF tests for all patients switching over would be the best practice approach for maintaining clinical care accuracy.
Use in Practice
Our experience with HMDs varies with the practice setting and the clinical use of perimetry. Adoption in general eyecare practices with limited or no medical management has been very positive. In these settings, the HMD is used mostly for screening and success is dependent upon implementation, training, the ease of set up and connectivity between devices. Patient acceptance is generally quite high. As mentioned, the HMD may also offer other pre-test procedures that improve office patient flow. Lower cost and small size offer an option for placing devices in multiple exam and testing rooms. Transitioning from an older device is not too much of a concern in this setting as most patients are healthy and have not been managed for chronic disease.
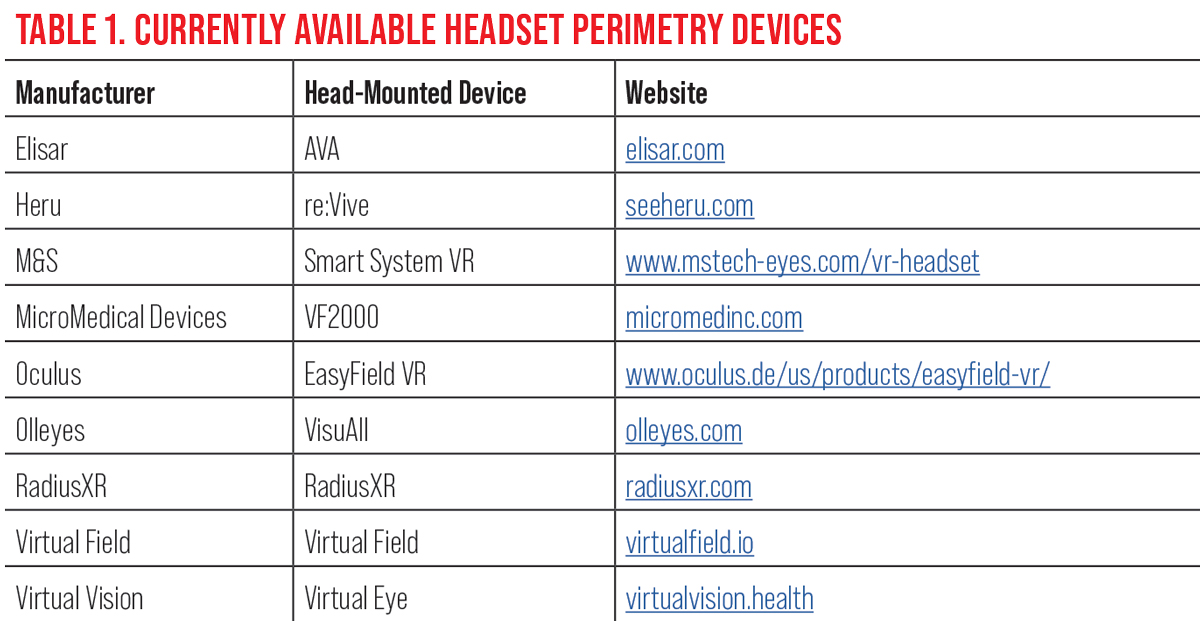 |
| Click table to enlarge. |
In offices with a large percentage of glaucoma patients, complete change over and exclusive implementation of any HMD is not practical at this time, in our opinion. However, there clearly is still a need and a role for HMDs in these medically focused offices. HMDs can be excellent for those patients who can no longer sit for a bowl device test and for those with mild, nonprogressive disease where an easy to administer HMD meets all testing needs. In cases where there is good similarity (judged on the shape, location, depth of scotoma) between the two test reports (patient would complete testing on both devices on the same or within a short time), we feel more confident in moving forward with an HMD. For those patients with more advanced disease or who are at higher risk of progression we’re generally more comfortable staying with a traditional test that has well-established progression analysis software. However, even in limited testing in our glaucoma practices, we’ve seen a clear and growing role for HMDs.
Takeaways
Overall, virtual reality or head-mounted perimetry has the potential to revolutionize visual field testing in our offices and clinics. The ergonomics and space saving features clearly offer advantages to many. However, caution should be exercised in these very early days of development and adoption. As clinicians, we must demand evidence of validation from the device manufacturers, and these studies should be published in the literature. The marketing approaches often address this superficially. Further long-term tests are also required to determine reproducibility and test-retest variability of this technology; without these, studies’ progression analysis cannot be appropriately incorporated into the summary reports.
Until that time, we suggest a careful and somewhat cautious integration of these devices. One thing is certain, the demand for innovation and change with traditional bowl perimetry is high, and the demand for high quality, reliable testing will drive the necessary improvements.
Dr. Hoepf is an assistant professor of clinical practice at Ohio State University, where she supervises optometry externs in the clinical setting at Columbus Ophthalmology Associates. She has no financial disclosures. Dr. Chaglasian is an associate professor at Illinois College of Optometry and chief of staff of the Illinois Eye Institute. He is also executive vice president of the Optometric Glaucoma Society. He has been a consultant to and/or received research funding from Bausch + Lomb, Carl Zeiss Meditec, Topcon and Optos.
1. Kimura T, Matsumoto C, Nomoto H. Comparison of head-mounted perimeter (imo) and Humphrey Field Analyzer. Clin Ophthalmol. 2019;13:501-513. 2. Olleyes - Virtual reality (VR) mobile perimeter and more. olleyes.com. Accessed January 18, 2024. 3. Montelongo M, Gonzalez A, Morgenstern F, et al. A virtual reality-based automated perimeter, device and pilot study. Transl Vis Sci Technol. 2021;10(3):20. 4. Ou Y. Checking visual fields using virtual reality. Rev Ophthalmol. 2021;28(3):64-6. 5. Jones L, Callaghan T, Campbell P, et al. Acceptability of a home-based visual field test (Eyecatcher) for glaucoma home monitoring; a qualityative study of patients’ views and experiences. BMJ Open. 2021;11:e043130. 6. Tsapakis S, Papaconstantinou D, Diagourtas A, et al. Visual field examination method using virtual reality glasses compared with the Humphrey perimeter. Clin Ophthalmol. 2017;11:1431-43. 7. Razeghinejad R, Gonzalez-Garcia A, Myers JS, Katz LJ. Preliminary report on a novel virtual reality perimeter compared with standard automated perimetry. J Glaucoma. 2021;30(1):17-23. 8. Johnson C, Sayed A, McSoley J, et al. Comparison of visual field test measurements with a novel approach on a wearable headset to standard automated perimetry. J Glaucoma. 2023; 32(8):647-57. 9. Groth SL, Linton EF, Brown EN, et al. Evaluation of virtual reality perimetry and standard automated perimetry in normal children. Transl Vis Sci Technol. 2023;12(1):6. 10. Chauhan BC, Garway-Heath DF, Goñi FJ, et al. Practical recommendations for measuring rates of visual field change in glaucoma. Br J Ophthalmology. 2008; 92(4):569-573. 11. Anderson AJ, Bedgood PA, George Kong YX, et al. Can home monitoring allow earlier detection of rapid visual field progression in glaucoma? Ophthalmology. 2017;124(12):1735-42. 12. Myers L, Hu R, Morgan LS, et al. A comparison of learning effects for standard automated perimetry, short-wavelength automated perimetry and frequency-doubling technology perimetry in healthy subjects. Invest Ophthalmol Vis Sci. 2014;55(13):5612. 13. Yousefi S, Goldbaum MH, Balasubramanian M, et al. Glaucoma progression detection in using structural retinal nerve fiber layer measurements and functional visual field points. IEEE Trans Biomed Eng. 2014;61(4):1143-54. 14. Heijl A, Patella VM, Bengsston B. The Field Analyzer Primer: Excellent Perimetry. 5th Ed. Carl Zeiss Meditec, Inc, 2021. 15. Nishida T, Eslani M, Weinreb RN, et al. Perimetric comparison between the IMOvifa and Humphrey Field Analyzer. J Glaucoma. 2023;32(2):85-92. 16. Nakai Y, Bessho K, Shono Y, Taoka K, Nakai Y. Comparison of imo and Humphrey field analyzer perimeters in glaucomatous eyes. Int J Ophthalmol. 2021;14(12):1882-7. 17. Goukon H, Hirasawa K, Kasahara M, Matsumura K, Shoji N. Comparison of Humphrey Field Analyzer and imo visual field test results in patients with glaucoma and pseudo-fixation loss. PLoS One. 2019;14(11):e0224711. 18. Wakayama A, Matsumoto C, Ayato Y, Shimomura Y. Comparison of monocular sensitivities measured with and without occlusion using the head-mounted perimeter imo. PLoS One. 2019;14(1). 19. Nishida T, Weinreb RN, Arias J, Vasile C, Moghimi S. Comparison of the TEMPO binocular perimeter and Humphrey field analyzer. Sci Rep. 2023;13(1):21189. |