 |
|
One reason advanced biological age may increase susceptibility of the optic nerve to damage is due to reduced ability to handle oxidative stress, a factor recognized to contribute to glaucomatous damage. Photo: Brian D. Fisher, OD. Click image to enlarge. |
The neurogenerative eye disease of glaucoma has been established to come with many risk factors; however, a new study is the first to both explore and establish a link between epigenetic age and glaucoma progression, rather than focusing on actual biological age.
To investigate this relationship, researchers from Bascom Palmer selected 100 primary open-angle glaucoma (POAG) patients with fast progression and 100 POAG patients with slow progression from a large database. Fast vs. slow progressors were determined by rates of change in standard automated perimetry mean deviation and retinal nerve fiber layer (RNFL) thickness. Four different “clocks” (i.e., testing protocols and criteria) were used to calculate epigenetic age from DNA methylation profiles obtained by blood samples.
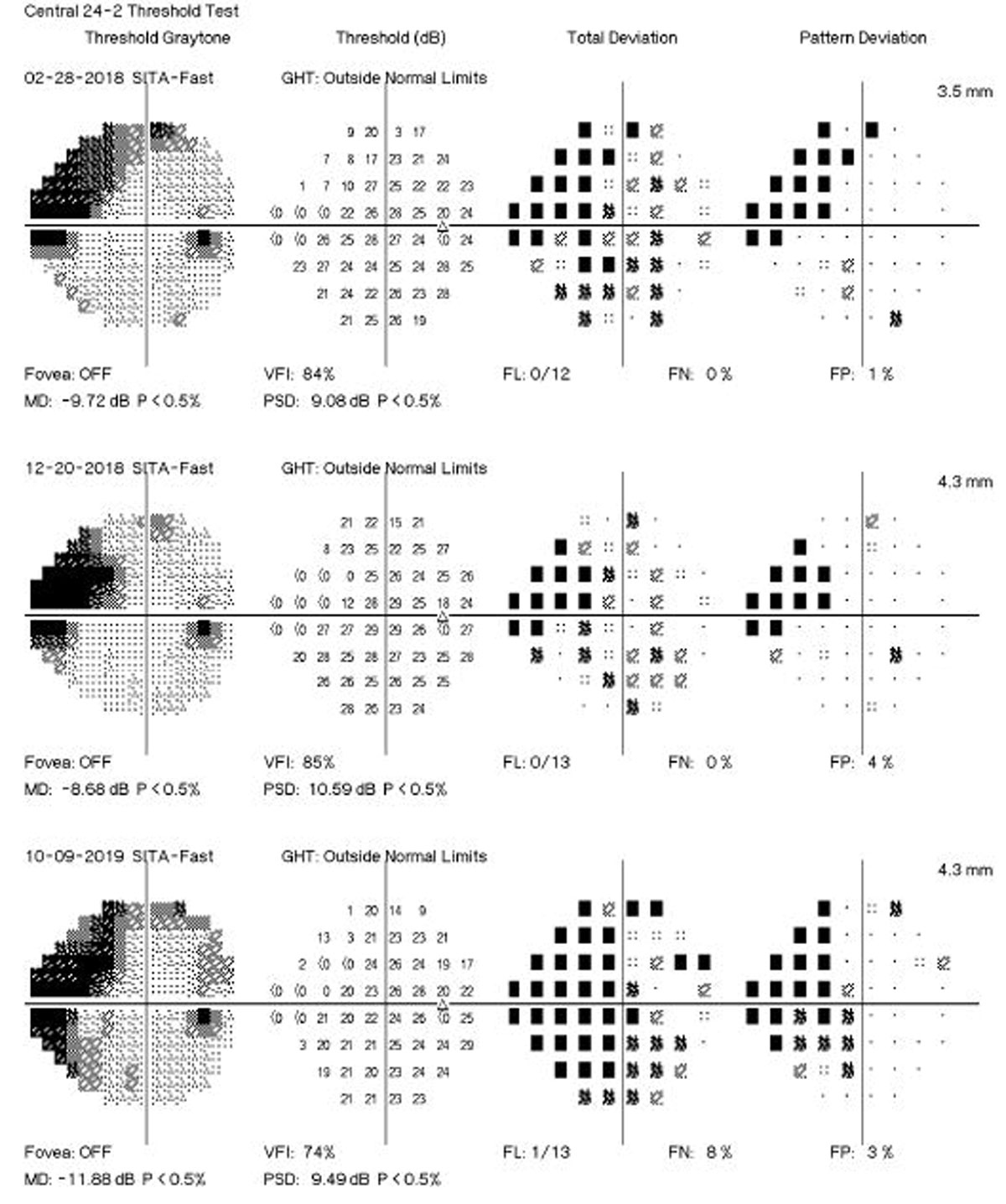
The mean rate of perimetry change in fast progressing eyes was -1.06dB/year, a marked increase from the mean rate of just -0.10dB/year for slow progressors. RNFL thickness displayed similar disparities, with fast progressors averaging change of -1.60µm/year vs. -0.76µm/year in slow progressors. Significantly greater age acceleration was observed in fast progressors via two of the epigenetic clocks. Multivariable models demonstrated that each year of age acceleration was associated with 15% higher odds of fast progression, even after adjustments for sex, race, intraocular pressure, central corneal thickness, baselined disease severity, smoking status and follow-up time. With the strongest association, this method of demonstrated fast progressors had a mean age acceleration of almost three years compared to slow progressors. The other clocks also showed significant associations with fast progressions, and the association between age acceleration and fast progression was stronger in subjects with relatively low intraocular pressure during follow-up.
Based on their results, the authors of the study write in their paper that “accelerated epigenetic aging was associated with faster glaucoma progression. These findings suggest that faster biological age, as reflected in DNA methylation, may increase optic nerve susceptibility to damage, highlighting epigenetic age as a potential prognostic biomarker.”
Upon discussion in the paper, the authors elaborate that the distinct contrast between rates of change in fast and slow progressors is clinically significant; over the follow-up period, fast progressors lost an average of 8.2dB in mean deviation in their fastest progressing eye, while slow progressors only 1.4dB—highlighting substantial differences in disease trajectory.
Due to the epigenetic acceleration of age affecting glaucoma progression, the authors argue that there is a potential for neuroprotective strategies targeting the aging processes to work as therapeutic agents in disease management. Precursors to NAD+, nicotinamide mononucleotide and nicotinamide have shown promise in animal studies, and nicotinamide has also improved inner retinal function in glaucoma patients.
Although more studies are needed to establish safety and efficacy of these compounds in humans, the authors believe that “these findings, coupled with our observations on epigenetic age acceleration, highlight the potential of targeting aging processes as a novel therapeutic approach in glaucoma management.”
| Click here for journal source. |
Medeiros FA, Varma A, Jammal AA, Tseng H, Scott WK. Accelerated epigenetic aging is associated with faster glaucoma progression: a DNA methylation study. medRxiv. October 14, 2024. [Epub ahead of print]. |


