 |
|
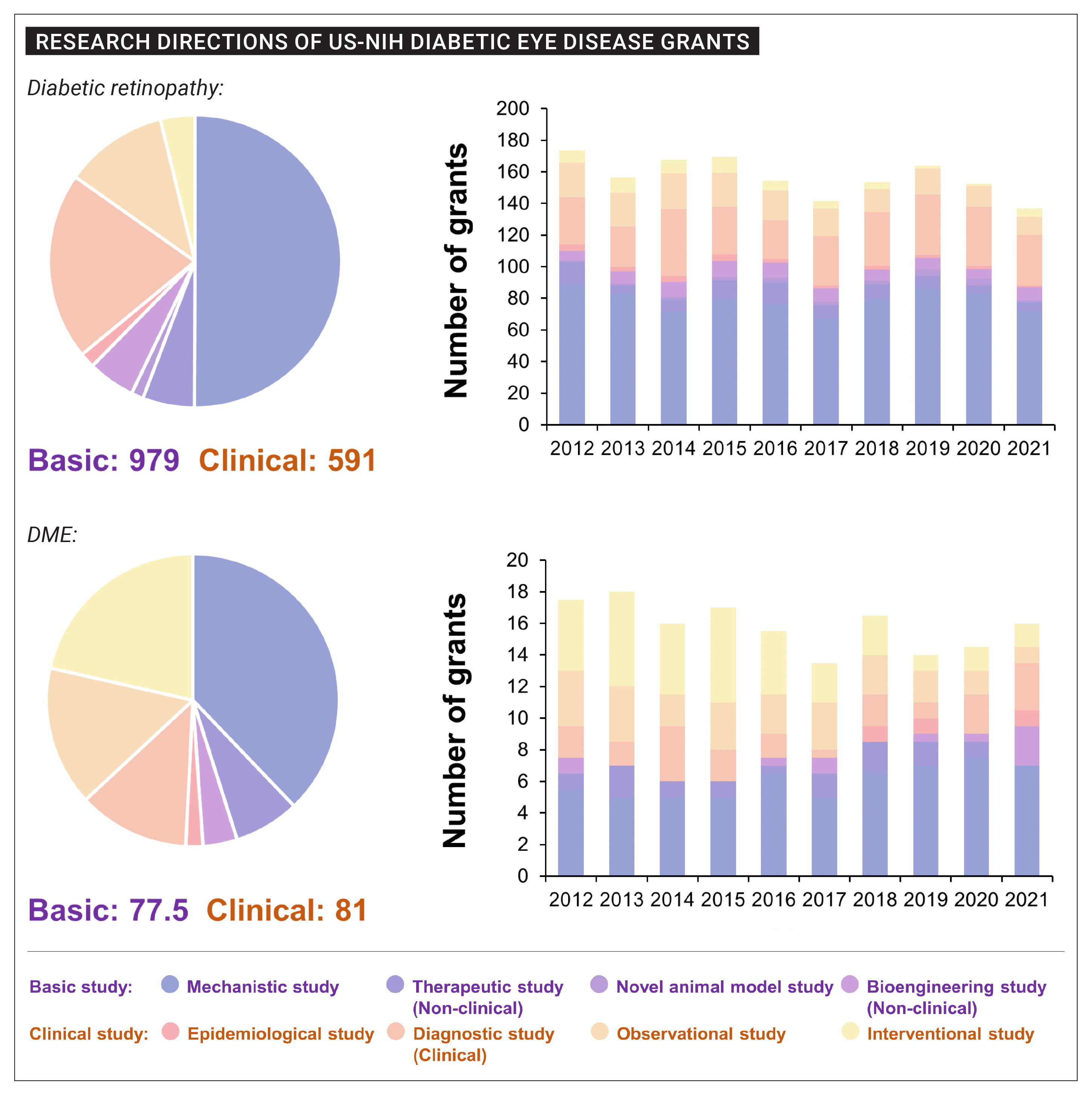
US-funded research in diabetic eye disease 2012-2021 was spread over a diverse range of study designs and purposes. Activities conducted toward diabetic retinopathy endpoints outnumbered those targeting DME roughly 10-fold. Photo: Yuan Y, et al. Neural Regen Res 2024;19:10:2310-2320. Click image to enlarge. |
Current estimates put the number of people with diabetes at 537 million worldwide as of 2021, with an additional 106 million more projected to be identified by 2030. Come 2045, the global diabetes population could reach 783 million individuals. The implications for optometrists and ophthalmologists are obvious, as cases of diabetic eye disease rise each year. How has the scientific community done at addressing this public health need? Results are mixed, with just a handful of new drug approvals despite an overwhelming amount of research projects funded by various governments.
In a recent analysis for a journal called Neural Regeneration Research, researchers conducted an evaluation of global diabetic eye disease research trends spanning 2012 to 2021 by collecting research grant information from seven representative countries: the United States, China, Japan, the United Kingdom, Spain, Germany and France. Industry-funded research was not included in this analysis.
Here are some of the key findings:
• Government funding sources bankrolled 2,288 research grants on diabetic eye disease between 2012 and 2021. Of these, diabetic retinopathy was the target of 89.5% studies while diabetic macular edema accounted for 9.3%.
• The United States National Institutes of Health led the world in diabetic eye disease research funding, underwriting 1,748 of the 2,288 studies, to the tune of $901.9 million, far exceeding the support of the other six study countries combined.
• In research output, the United States and China published 17.5% and 19.6% of papers, respectively. The United States received 22.6% of total citations.
• A total of 415 clinical trials of new therapeutic agents were registered during the period. Of these, the United States registered 157 trials, followed by 51 for China. Diabetic macular edema was the target of 58.2% and diabetic retinopathy 39.8%. Angiogenesis inhibitors accounted for 85 trials, while 65 tested VEGF inhibitors. Other drug categories included anti-inflammatory drugs (41 trials), metabolic regulators (16 trials), neuroprotective drugs (11 trials), IOP-lowering drugs (nine trials), antioxidant drugs (seven trials) and anti-infectious drugs (four trials).
• Despite 1,830 drug candidates being tested for diabetic eye disease applications worldwide, only three new agents were approved (0.02% approval rate) during the period, all in Europe: Eylea, Duloxetine Mylan and Byooviz. The FDA had approved Eylea for AMD in November 2011, just prior to the study period, and the drug did earn new indications for diabetic eye disease during the period in question.
The researchers concluded in their paper that basic and translational research for diabetic eye disease has been lacking in the past decade. They wrote, “Because of the growing demand for diabetic eye disease research, the global scientific community needs to make a substantial commitment to basic and clinic research in diabetic eye disease, particularly diabetic cataract and diabetic glaucoma, through active academic investigation, global collaboration and novel drug development.”
They also emphasized the need to recognize “the intricate interplay between data, collaboration, funding and research methodologies,” saying that these aspects are “essential to advancing our understanding of this field.”
Yuan Y, Ji S, Song Y, et al. Global trends in diabetic eye disease research from 2012 to 2021. Neural Regen Res 2024;19:10:2310-2320. |


