
As providers of primary eye care, optometrists regularly examine and diagnose pat- ients with glaucoma and then manage these patients on a long-term basis. Gonioscopy is central to the glaucoma exam, and may be the most important factor in making a correct diagnosis.1 The most common cause of incorrect glaucoma diagnosis is omission of gonioscopy, particularly when slit lamp examination shows no sign of angle closure or inflammation, yet the angle is not open.1
After diagnosing and following the patient over time, periodically repeat gonioscopy to ensure there has been no angle progression.1,2 Intermittent repeat gonioscopy is warranted on a patient suffering from age-related lens changes (particularly in hyperopes), when the anterior chamber appears to be-come more shallow, after filtering surgery (argon laser trabeculoplasty [ALT], selective laser trabeculoplasty [SLT] or laser peripheral iridotomy [LPI]), and after a detected increase in IOP.2
Several grading systems for anterior chamber (AC) anatomy exist. Some systems allow for highly accurate angle evaluation, while others are prone to error. This article re-views these systems and presents a clinician-friendly alternative.
 Gonioscopy Types
Gonioscopy Types
Gonioscopy allows for visualization of the anterior chamber angle structures even when direct visualization of these structures is prevented by internal reflection. There are two basic types of gonioscopy: direct and indirect.
Direct gonioscopy is accomplished by placing a Koeppe lens on the recumbent patient. I believe this method is not clinician friendly, and thus, should not be performed routinely.
Indirect gonioscopy uses mirrors to visualize angle structures. Common lenses used for this method include the Goldmann three-mirror lens, the Zeiss four-mirror lens, and other variants.
 The Goldmann three-mirror lens requires a clear fluid to fill the space between the central lens and anterior corneal surface. Suction helps to keep the lens in place. However, the suction can distort the anatomical relationships of the angle. Once completed, the patient may require irrigation to remove the viscous interface fluid.
The Goldmann three-mirror lens requires a clear fluid to fill the space between the central lens and anterior corneal surface. Suction helps to keep the lens in place. However, the suction can distort the anatomical relationships of the angle. Once completed, the patient may require irrigation to remove the viscous interface fluid.
Because of the ease of keeping the lens on the eye, novices generally favor this method. In cases of convex iris, the viewer can see over the hill by having the patient look in the direction of the mirror being viewed.1
The Zeiss four-mirror goniolens uses the tear film for the lens/cor- neal interface. It is more difficult to keep the lens in place when using this technique. Once mastered, however, this is the most patient- and practitioner-friendly gonioscopy technique.
The lens can be placed on the eye in the diamond or square orientation. Ultimately, I have found that the square orientation is both easier to position and better for recording angle structures.
 Angle Structures
Angle Structures
From cornea to iris, the angle structures visible on gonioscopy include Schwalbes line, trabecular meshwork (TM), scleral spur and ciliary body base (figure 1).
Schwalbes line. This is the peripheral limit of Descements membrane. When prominent in appearance, it is called posterior embryotoxin (figure 2).1
Schwalbes line creates a shelf on which pigment can deposit. This pigment deposition is termed the Sampaolesi line. On occasion, this line can be so dense that it is mistaken for pigmented TM in an angle that is actually closed.1
 Pressure on the cornea by the goniolens (compression/indentation gonioscopy) can push the angle open and help determine the true angle configuration. Also, pigment layered on Schwalbes line tends to be more granular and less uniform in distribution, while pigment in the trabecular meshwork has more of a brown sugar appearance and a more uniform distribution.1
Pressure on the cornea by the goniolens (compression/indentation gonioscopy) can push the angle open and help determine the true angle configuration. Also, pigment layered on Schwalbes line tends to be more granular and less uniform in distribution, while pigment in the trabecular meshwork has more of a brown sugar appearance and a more uniform distribution.1
Trabecular meshwork. The TM is the next structure seen in the angle. It is divided into anterior non-pigmented and posterior pigmented portions.
The pigmented TM is where filtration occurs into the underlying Schlemms canal. It must be accessible for aqueous outflow to occur and intraocular pressure to remain relatively stable.
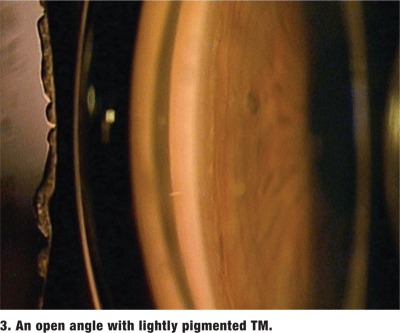
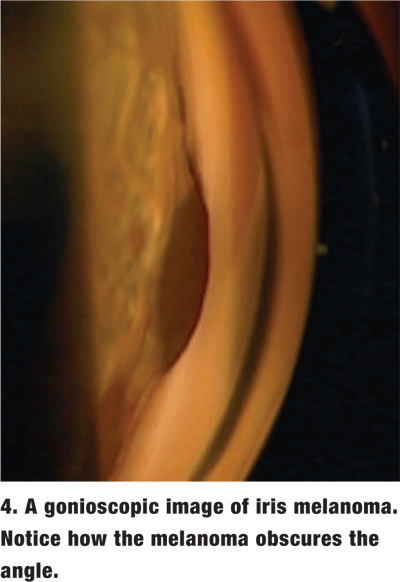
 The amount of pigment in the TM varies and is graded on a scale from 0 (no pigment) to 4 (very heavy pigment) (figure 3). More pigment generally is seen in the TM of darkly pigmented irises and in older individuals. Dense pigment in the TM, especially in the superior angle, is also a distinguishing feature of pigment dispersion/ pigmentary glaucoma as well as pseudoexfoliation of the lens capsule. Increased amounts of trabecular pigment may also be seen after trauma, surgery, inflammation and hyphema, and in malignant melanoma (figure 4).2
The amount of pigment in the TM varies and is graded on a scale from 0 (no pigment) to 4 (very heavy pigment) (figure 3). More pigment generally is seen in the TM of darkly pigmented irises and in older individuals. Dense pigment in the TM, especially in the superior angle, is also a distinguishing feature of pigment dispersion/ pigmentary glaucoma as well as pseudoexfoliation of the lens capsule. Increased amounts of trabecular pigment may also be seen after trauma, surgery, inflammation and hyphema, and in malignant melanoma (figure 4).2
Scleral spur. This structure is the prominent internal extension of the sclera. It appears as a white line between the ciliary base and the pigmented zone of the TM.2 TM beams insert into the anterior edge of the spur, and ciliary muscle fibers insert into the posterior edge.1
Ciliary body base. This appears as a gray or brown band between the scleral spur and iris root. This band may not be visible in narrow angles. It may appear as a thin band in deeper angles or as a wide band in very deep angles. In cases of angle recession, the band may be extremely wide.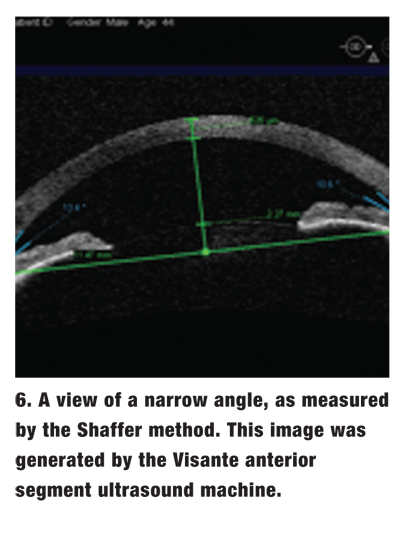 Grading Systems and Procedures
Grading Systems and Procedures
There are several grading systems for assessing AC depth.
Van Herick method. This relatively simple method is done as part of the slit lamp exam. It involves grading the anterior chamber depth compared to corneal thickness when the light housing is set at 60. The angle is considered closed if the iris is touching the corneal endothelium (figure 5).
The Van Herick method is not an adequate substitute for gonioscopy. Angles that appear open by Van Herick grading may actually be too narrow and are in danger of closure, as seen on gonioscopy.3 Even if the angle is open, important information, such as peripheral anterior synechiae (PAS), angle recession and neovascularization, can be missed in the absence of a gonioscopic examination.
Shaffer method. In this system, the observer estimates the geometric angle created between the iris and the corneoscleral wall (figure 6).

Spaeth method. This is an elegant system for describing the AC angle. This method expands the Shaffer method to include a determination of the shape of the peripheral angle and the insertion of the iris root. 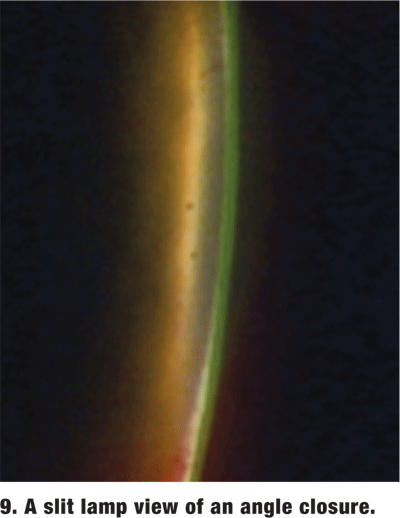 All of the aforementioned grading systems are meant to determine which angles may close and which may not. Therefore, gonioscopy ultimately aims to address the following questions:
All of the aforementioned grading systems are meant to determine which angles may close and which may not. Therefore, gonioscopy ultimately aims to address the following questions:
Is the angle open or closed?
If it is open, is it in danger of closure?
What is the deepest visible structure?
Is the approach of the iris to ciliary body base steep or flat?
Are there any other noteworthy findings, such as pigment in the TM, PAS, neovascularization or tumors?
When performing gonioscopy, be careful not to apply too much pressure. If too much pressure is applied, folds in the cornea may appear (figure 7). Also, to obtain a view of the angle, the interface of the four-mirror gonio-lens and the corneal surface must be complete with a uniform tear layer. If it is not complete, youll see a bubble at this interface and no angle structures (figure 8). 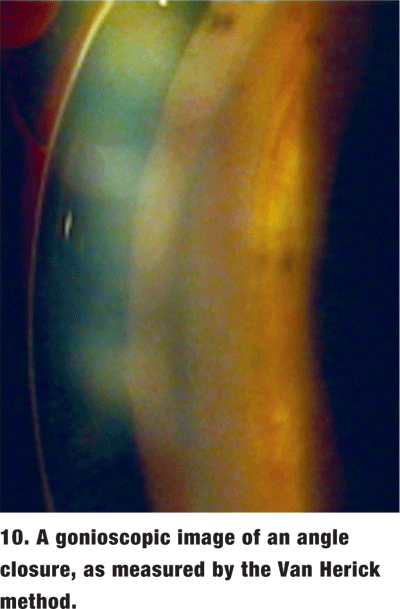 To fix this, gently slide the goniolens away from the bubble to complete the interface and permit visualization. Perform this action while looking at the central goniolens through the slit lamp. Once you determine the status of the angle, make certain to carefully look for any abnormalities.
To fix this, gently slide the goniolens away from the bubble to complete the interface and permit visualization. Perform this action while looking at the central goniolens through the slit lamp. Once you determine the status of the angle, make certain to carefully look for any abnormalities.
Abnormalities Detected by Gonioscopy
Acute angle closure. This rarely seen, yet potentially devastating, ocular pathology presents with the classic steamy cornea, mid-dilated pupil, shallow AC, markedly elevated IOP, brow ache, and sometimes nausea (figures 9 and 10). This is an ocular emergency. In this event, gonioscopy may be difficult to perform due to patient discomfort and corneal edema.
Topical glycerin may help clear the edema to allow gonioscopic visualization of the angle. Compression/indentation gonioscopy may help break the attack.
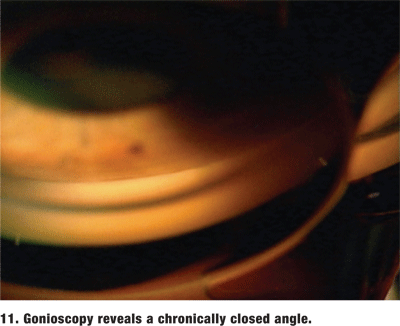 Always perform gonioscopy on the fellow eye to try to confirm the diagnosis and determine its risk for closure.1,2 In almost every case, the fellow phakic eye will also be at risk for angle closure. If the angle is deep in the fellow eye, reconsider the primary diagnosis. Do not forget that patients with either chronic angle closure or intermittent angle closure will often present with the typical history of acute angle closure without any visible symptoms (figure 11).
Always perform gonioscopy on the fellow eye to try to confirm the diagnosis and determine its risk for closure.1,2 In almost every case, the fellow phakic eye will also be at risk for angle closure. If the angle is deep in the fellow eye, reconsider the primary diagnosis. Do not forget that patients with either chronic angle closure or intermittent angle closure will often present with the typical history of acute angle closure without any visible symptoms (figure 11).
Plateau iris. This is an angle configuration created when the ciliary processes push the peripheral iris forward. Suspect plateau iris if the central AC depth seems unusually deep and the iris plane appears flat in the presence of angle closure.2
 Be sure to distinguish between a normal angle finding, iris processes and PAS. Iris processes are lacy cords of uveal tissue that run from the peripheral iris to the TB (figure 12).1 PAS are adhesions of the iris to the TM. They can be focal or broad. PAS prevent movement of the peripheral iris on compression/indentation gonioscopic examination.
Be sure to distinguish between a normal angle finding, iris processes and PAS. Iris processes are lacy cords of uveal tissue that run from the peripheral iris to the TB (figure 12).1 PAS are adhesions of the iris to the TM. They can be focal or broad. PAS prevent movement of the peripheral iris on compression/indentation gonioscopic examination.
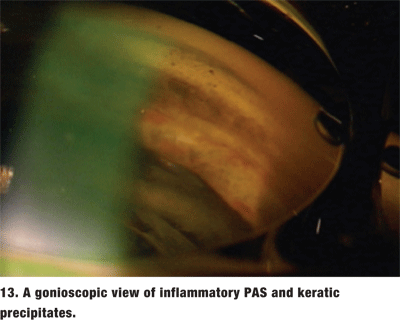
The location of the PAS can provide a clue to their etiology. If the PAS are the result of an angle closure, they tend to be in the superior angle. Inflammatory PAS are broad-based and tend to be in the inferior angle (figure 13). Focal, or tooth-shaped PAS may be a result of ALT or SLT. PAS that are secondary to iridocorneal epitheliopathies may insert anterior to Schwalbes line.1
 Blunt Trauma
Blunt Trauma
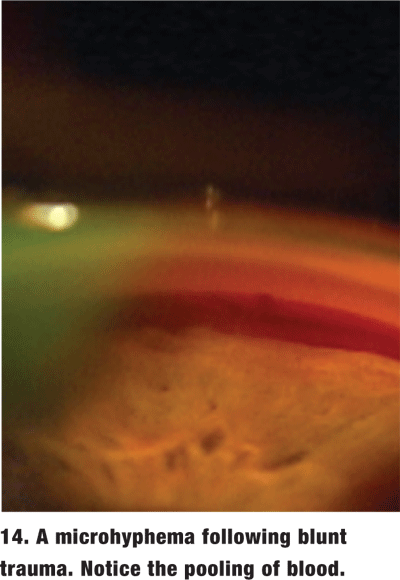
Gonioscopy is often necessary to look for damage to the angle in a patient who has suffered blunt trauma. When the patient has an obvious hyphema or open globe, delay gonioscopy until the patient has stabilized. However, in the absence of frank hyphema, gonioscopy is essential at the initial exam to rule out the possibility of a microhyphema (figure 14). The treatment for microhyphema is virtually identical to that for hyphema and requires patient activity restrictions for a timely and accurate diagnosis.
Consider angle recession if you observe an abnormally wide ciliary body band; increased prominence of the scleral spur; torn iris processes; or a marked variation in the width of the ciliary base and angle depth in different quadrants of the same eye. To more accurately determine whether an angle is recessed, compare quadrants of the involved eye with the same quadrants of the fellow eye.2
Also, evaluate the angle for the presence of abnormal blood vessels, as these can signal the onset of devastating ocular complications from ischemia. These may be seen in patients with diabetes, vascular occlusion, or chronic retinal detachment. Neovascularization also can occur in conjunction with Fuchs heterochromic iridocyclitis, uveitis and pseudoexfoliation.2
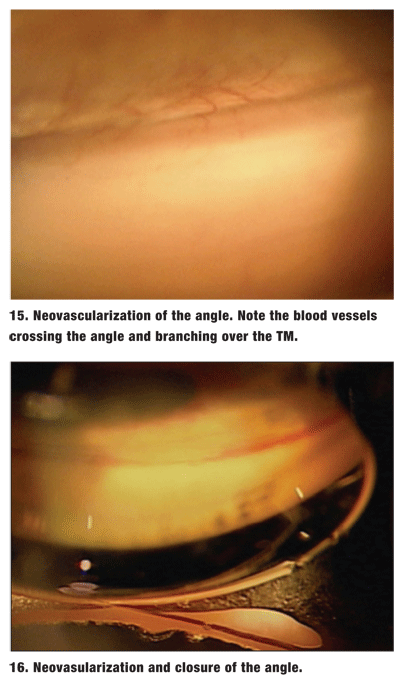 These abnormal vessels must be differentiated from normal radial iris vessels and the arterial circle of the ciliary body. Normal vessels are oriented radially along the iris and circumferentially in the ciliary body base. Abnormal angle vessels cross the scleral spur to the TM. They may appear trunk-like over the ciliary body base and scleral spur, then branch out over the TM (figure 15).2 The TM may take on a reddish hue. This appearence should be differentiated from the blood in Schlemms canal caused by elevated episcleral pressure.
These abnormal vessels must be differentiated from normal radial iris vessels and the arterial circle of the ciliary body. Normal vessels are oriented radially along the iris and circumferentially in the ciliary body base. Abnormal angle vessels cross the scleral spur to the TM. They may appear trunk-like over the ciliary body base and scleral spur, then branch out over the TM (figure 15).2 The TM may take on a reddish hue. This appearence should be differentiated from the blood in Schlemms canal caused by elevated episcleral pressure.
These abnormal vessels stop at the TM and cannot grow over normal corneal endothelium. The fibrovascular membrane can cause both PAS and neovascular glaucoma (figure 16).2
As providers of primary eye care, doctors of optometry must master both gonioscopy procedures and anterior chamber anatomy. Gonioscopy is an essential skill for the diagnosis and management of glaucoma. After learning this relatively easy technique, you can quickly and efficiently include the procedure in the basic exam without slowing patient flow or inconveniencing patients.

Dr. Findley is the center director for Commonwealth Eye Surgery, a comanagement practice in
1. Ritch R, Sheilds B, Krupin T. The Glaucomas, vol. 1. St.Louis: Mosby Co.;1989:345-60.
2. Cantor LB, 2002-2003 Basic and Clinical Science Course (BCSC) Section 10: Glaucoma.
3. Rhee DJ, Pyfer MF. The Wills Eye Manual. 3rd ed.

