Various insults can occur to the optic nerve and manifest ophthalmoscopically as pallor, atrophy, cupping and notching. By far, the most common optic nerve affliction is glaucoma.
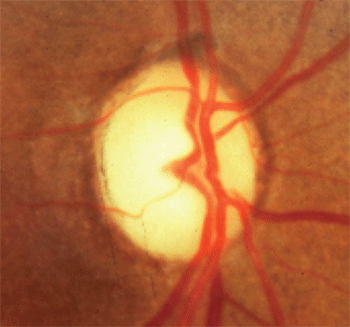
1. Optic disc pallor in excess of cupping.
Glaucoma presents with a very characteristic cavernous atrophy, commonly termed “cupping” or “notching.” This distinctive neuropathy presents with enlargement of the optic disc cup—preferentially at the inferior and superior regions—with an enlarged vertical cupping pattern and compromise or obliteration of the neuroretinal rim.
In this chronic disease, there typically is an absence of pallor. The rim tissue remains well perfused, although focal damage occurs to the superior and inferior neuroretinal rim.
Occasionally, patients will present with glaucomatous-type optic nerves and exhibit additional features, such as pallor, which indicate the presence of another condition entirely or an entity in addition to glaucoma. There are also several clinical entities and situations in which the optic nerve can be compromised and superficially resemble glaucoma. It is imperative to correctly differentiate these non-glaucomatous neuropathies from glaucoma.
Is There Optic Disc Pallor?
Identifying optic disc pallor sometimes is difficult when the condition is subtle, or there are media and cataract issues impairing judgment. It can also be challenging to differentiate true disc pallor from mimics. For example, after cataract extraction, there is a loss of the light-attenuating properties of the natural lens. The disc can appear pale, but there is no nerve dysfunction—this is known as “pseudophakic pseudopallor,” and can be quite disconcerting and misleading.1
In unilateral cases, it helps to photograph each disc and compare them side by side. When doing so, optic disc pallor in one eye can be more easily identified, even when it is subtle.
Optic atrophy, manifesting as optic disc pallor, rarely exists in isolation. As such, it is imperative to look for associated optic nerve dysfunction to separate the mimicking conditions from true optic atrophy.
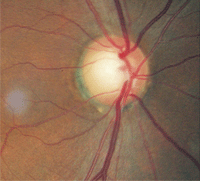
2. Relative disc pallor, O.D. (left) compared to O.S. (right). The greater pallor in the left eye indicates optic atrophy.
Associations that would indicate true optic atrophy include visual field loss, visual acuity reduction, dyschromatopsia, relative afferent pupil defect (when unilateral or asymmetric) and retinal nerve fiber layer damage. In the absence of any such abnormality, you must question whether the patient has true optic atrophy.
Optic disc pallor, indicating optic atrophy, is more a finding than a diagnosis. When identified, an explanation or underlying cause must be sought. The term pallor should never be used cavalierly. That is, never write that you see or suspect disc pallor on a patient’s chart unless an investigation into the cause is undertaken. If you don’t have an explanation for disc pallor, you must obtain proper neuroimaging. Failure to investigate any recorded finding of disc pallor is akin to writing on a patient chart, “I think that the patient may have a brain tumor … but I’m choosing to do nothing about it.”
There are numerous causes of optic atrophy, including direct compression of the nerve or chiasm by a mass lesion, infarction, trauma, toxicity inflammation, infiltration, and metabolic dysfunction, to name a few.
Compressive Lesions That Cause Disc Pallor
Compressive neuropathy by a mass lesion often can result in disc pallor and optic atrophy. Prolonged compression results in optic atrophy, which may present with pallor and/or cupping of the nerve head. Optociliary collateral vessels may be noted at the disc margin.2,3 Frequently, there will be increased progressive cupping of the optic nerve head, somewhat similar to that seen in glaucoma.4-6 The main differentiating factor from glaucomatous optic atrophy is the pallor of the remaining neuroretinal rim in compressive neuropathy.
Also, there is more significant neuroretinal rim compromise in the form of notching that occurs in glaucoma but not in compressive lesions, where cup increase is more symmetrical with pallor. Associated field defects with compressive lesions include central scotomas, arcuate or altitudinal defects, paracentral scotomas, field constriction and defects respecting the vertical hemianopic line.

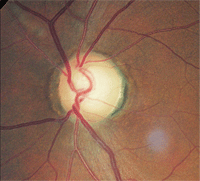
3. The same patient as above had a bitemporal visual field defect secondary to chiasmal tumor.
Direct impingement of the optic nerve is the mechanism of compromise in compressive optic neuropathy. Most often, this stems from a space-occupying mass within the orbit; although it may occur at the optic chiasm or suprasellar cistern. In these cases, the patient likely will have vertically oriented visual field loss.
Conditions associated with compressive optic neuropathy include: a dolichoectatic carotid artery; mucocele; thyroid ophthalmopathy; neoplasms, including optic nerve gliomas, nerve sheath meningiomas, dermoid cysts, neurilemmomas (schwannomas) and orbital metastases; vascular anomalies, such as cavernous hemangioma, lymphangioma, simple venous varix, arteriovenous malformation, carotid aneurysm and carotid cavernous fistula; inflammations, including orbital cellulitis and orbital pseudotumor; Wegener’s granulomatosis; and subperiosteal or intraorbital hemorrhage.7-16
The pathogenesis of this condition is mechanical compression of the nerve that induces stagnation of axoplasmic flow within the individual neurons—in both the slow and fast phases of axoplasmic transport. This axoplasmic stasis causes subsequent swelling of the axons as well as leakage of intracellular fluids, lipids and proteins into the extracellular space of the prelaminar optic disc.
Vascular changes occur secondarily, as venous drainage via the central retinal vein is impeded by continued mechanical stress. As this process persists, hypoxia and disorganization of the normal neural matrix follows. If left untreated, optic atrophy will ultimately ensue. The atrophy may be total or sectoral.
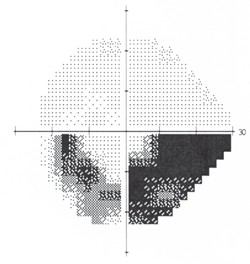
4. Inferior arcuate defect that was consistent with glaucoma.
In 1998, David Greenfield, M.D., and colleagues published a seminal paper discussing which patients with glaucoma need neuroimaging.17 In their retrospective analysis, they compared glaucoma patients who had normal intraocular pressure (IOP) and had undergone neuroimaging as part of their evaluation (due to the normal IOP) with patients in whom compressive lesions were diagnosed.
They noted that none of the patients diagnosed with normal-tension glaucoma had neuroradiologic evidence of a mass lesion in the anterior visual pathway. They also found that, when compared to the control group with mass lesions, those patients with glaucoma were older as well as had better visual acuity, greater vertical loss of neuroretinal rim, more frequent disc hemorrhages, less neuroretinal rim pallor and more nerve fiber bundle defects aligned along the horizontal midline.
In contrast, those patients with mass lesions of the anterior visual pathway typically were under the age of 50 and exhibited a visual acuity of less than 20/40, vertically aligned visual field defects, and optic disc pallor in excess of cupping (figure 1). They concluded that younger age, lower levels of visual acuity, vertically aligned visual field defects and neuroretinal rim pallor were more indicative of a compressive mass lesion than glaucoma.
Case Examples
Case 1. A 56-year-old black male presented for a glaucoma consultation. He had been diagnosed with glaucoma six years earlier in Nigeria, but never received treatment.
His best-corrected visual acuity was 20/30 O.D. and light perception O.S. His pupils were equal and reactive with a left relative afferent defect. IOP was 30mm Hg O.D. and 23mm Hg O.S. Biomicroscopy revealed no abnormalities, and gonioscopy demonstrated normal, open anterior chamber angles.
Dilated fundus examination revealed optic disc cupping of 0.75/0.75 O.D. and 0.80/0.80 O.S. There was distinct damage to the left neuroretinal rim. Nerve fiber layer analysis via scanning laser polarimetry was abnormal in the left eye. However, what was most notable was that the remaining left neuroretinal rim was pale in comparison to that in the fellow eye (figure 2).
The patient clearly had optic atrophy in his left eye. Furthermore, his acuity of light perception was not consistent with optic disc cupping of 0.80/0.80, if glaucoma were the sole cause. At this point, he was diagnosed with glaucoma as one potential condition and suspected of having an additional neuropathy as well.
Threshold perimetry revealed a bitemporal visual field defect that respected the vertical hemianopic line of the right eye and crossed over to involve fixation in the left eye (figure 3). This was consistent with a chiasmal tumor. Magnetic resonance imaging (MRI) confirmed the diagnosis.
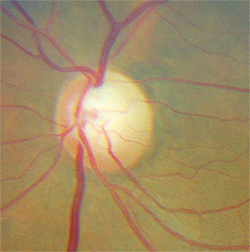
5. The same patient as above demonstrated superior rim notching and pallor.
This patient had a tumor as well as glaucoma. The key diagnostic finding was disc pallor and visual acuity loss that was much worse than would be expected from glaucoma, as well as vertically oriented visual field loss.
Case 2. A 53-year-old black female was referred for suspicion of glaucoma. She had no visual or ocular complaints. Her best-corrected visual acuity was 20/20 in each eye.
Pupils were reactive, with a relative afferent defect in the left eye. Color vision testing was normal. There were no biomicroscopic abnormalities, and her anterior chamber angles were open gonioscopically. Intraocular pressure measured 30mm Hg O.D. and 32mm Hg O.S.
Threshold perimetry revealed a full and normal field O.D. and a dense inferior arcuate defect O.S., which was consistent with glaucoma (figure 4). The dilated examination raised concern. Her right optic disc was pink, distinct and normal. However, the patient’s left nerve, while demonstrating notching of the superior temporal neuroretinal rim, was also pale in this region (figure 5).
Though her acuity and color vision were normal and her IOP clearly was in a glaucomatous range, the unilaterality and the presence of disc pallor in her left eye raised the suspicion of an optic nerve compressive tumor. The patient underwent MRI of the orbits and chiasm—both with and without contrast—which turned out to be normal.
Despite the suspicious disc appearance, the patient has nothing more than glaucoma and continues to do well with topical glaucoma therapy.
Consider Retinal Causes
Case 3. A 72-year-old Indian male was referred for a glaucoma consultation. He had long been a suspect for normal-tension glaucoma based upon slightly asymmetrical disc cupping and visual field loss that was observed several years earlier.
He had been seen by a number of doctors in a university-based setting. He had received glaucoma treatment intermittently, and his file revealed that no medication trial ever seemed to lower his IOP below its untreated range of 12mm Hg to 14mm Hg.
His medical history was significant for controlled hypertension and hypercholesterolemia. After reviewing the patient’s past history and seemingly ineffective medical therapy, one previous doctor suggested monitoring the patient without treatment. Most recently, another doctor in the facility had noted superior disc pallor in the left eye and sought another opinion.
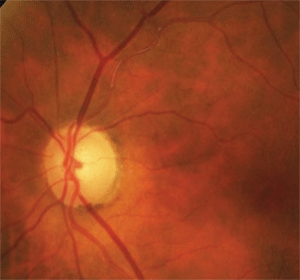
6. The superior temporal disc pallor and sclerotic, occluded arteriole were due to an old
asymptomatic branch retinal artery occlusion.
The patient’s best-corrected visual acuity was 20/20 O.D. and O.S. There was no relative afferent pupillary defect in either eye. The patient had no visual or ocular complaints, but understood that he was a glaucoma suspect due to what had been explained to him over the past several years.
Analysis of past threshold perimetry revealed an unchanging inferior arcuate scotoma in the left eye and a normal visual field in the right eye. Dilated fundus inspection of the optic nerves revealed a cup-to-disc ratio of 0.6 x 0.5 O.U. The right optic disc was normal without pallor or glaucomatous changes. There was no focal compromise of the neuroretinal rim of the left eye, which would explain the visual field loss if glaucoma were the cause. However, there was pallor of the superior disc of the left eye, which corresponded to the field loss. Thus, the patient had disc pallor in excess of cupping, pointing to a cause other than glaucoma.
To that end, careful inspection of the adjacent retinal vasculature revealed sclerosis of the superior temporal arteriole prior to and extending beyond the first bifurcation (figure 6). Additionally, there were faint retinal collateral vessels in the superior temporal area that were indicative of a previous retinal vascular occlusion. In this case, the patient’s field loss and disc pallor were due to an old asymptomatic branch retinal artery occlusion.
Approaching Disc Pallor
When optic disc pallor is seen, look to corroborate the finding with optic nerve dysfunction. Be sure to perform color vision testing, pupil inspection, threshold perimetry, best-corrected visual acuity measurement and retinal nerve fiber layer evaluation. These tests can guide you to the location and nature of the problem.
Patient history is crucial. Verify if the patient is aware of any changes. If there is vision loss, try to ascertain whether the reduction was gradual (indicative of compressive lesions) or abrupt (suggestive of optic nerve or retinal infarct). Identify if there was pain associated with vision loss (such as that seen in optic neuritis) or if the condition was painless (consistent with a non-arteritic anterior ischemic optic neuropathy). Finally, carefully review the patient’s past and present medical history for any possible contributory conditions, such as multiple sclerosis, thyroid dysfunction or cancer.
Should optic nerve compression be suspected, MRI of the orbits and chiasm, with and without contrast, should be ordered. Preferably, this imaging should be performed in a closed unit, because the detail will be better. When chiasmal or anterior visual pathway compression is suspected, imaging of the orbits and chiasm will be more revealing than that of the brain.
Bear in mind that optic disc pallor is more of a finding than a diagnosis and that a cause must be sought. Never record disc pallor in a patient’s file without first explaining or acting upon this finding. Carefully differentiate disc pallor from glaucomatous atrophy.
Always remember that pallor in excess of cupping is indicative of something other than—or in addition to—glaucoma, but that nothing notches a nerve like glaucoma.
Dr. Sowka is a Professor at Nova Southeastern University College of Optometry in Fort Lauderdale, Fla. He is also Chief of Advanced Care and Director of Glaucoma Services at The Eye Care Institute of Nova Southeastern University.
1. Snydacker D: The normal optic disc. Ophthalmoscopic and photographic studies. Am J Ophthalmol 1964; 58:958-964. Wilhelm H, Dörr S, Paulsen F, et al. Early symptoms and findings in optic nerve meningiomas. Klin Monbl Augenheilkd. 2009 Nov;226(11):869-74.
3. Rebolleda G, Corcóstegui J, Arruabarrena C, et al. Optociliary shunt vessels in compressive optic neuropathy by the intracranial internal carotid artery. Eur J Ophthalmol. 2008 Mar-Apr;18(2):316-9.
4. Roodhooft JM. Nonglaucomatous optic disk atrophy and excavation in the elderly. Bull Soc Belge Ophtalmol. 2003;(287):45-9.5. Hokazono K, Moura FC, Monteiro ML. 5. Optic nerve meningioma mimicking progression of glaucomatous axonal damage: a case report. Arq Bras Oftalmol. 2008 Sep-Oct;71(5):725-8.
6. Greenfield DS. Glaucomatous versus nonglaucomatous optic disc cupping: clinical differentiation. Semin Ophthalmol. 1999 Jun;14(2):95-108.
7. Rosen N, Ben Simon GJ. Orbital decompression in thyroid related orbitopathy. Pediatr Endocrinol Rev. 2010 Mar;7(Suppl 2):217-21.
8. Goh MS, McNab AA. Orbital decompression in Graves’ orbitopathy: efficacy and safety. Intern Med J. 2005 Oct;35(10):586-91.
9. Robert PY, Camezind P, Adenis JP. Complications of dysthyroid ophthalmopathy. J Fr Ophtalmol. 2004 Sep;27(7):819-21.
10. Oono S, Kurimoto T, Fukazawa K, Mimura O. Compressive optic neuropathy caused by a paranasal sinus cyst of Wegener’s granulomatosis. Jpn J Ophthalmol. 2007 Nov-Dec;51(6):480-1.
11. Chua CN, Alhady M, Ngo CT, et al. Solitary nasal neurofibroma presenting as compressive optic neuropathy. Eye (Lond). 2006 Dec;20(12):1406-8.
12. Mansour AM, Salti HI. Multiple myeloma presenting with optic nerve compression. Eye (Lond). 2001 Dec;15(Pt 6):802-4.
13. Wu W, Sun MT, Cannon PS, et al. Recovery of visual function in a patient with an onodi cell mucocele compressive optic neuropathy who had a 5-week interval between onset and surgical intervention: a case report. J Ophthalmol. 2010;2010:483056. Epub 2010 Oct 12.
14. George JL, Marchal JC. Orbital tumors in children: clinical examination, imaging, specific progression. Neurochirurgie. 2010 Apr-Jun;56(2-3):244-8.
15. Aakalu VK, Ahmad AZ. Wegener granulomatosis causing compressive optic neuropathy in a child. Ophthal Plast Reconstr Surg. 2009 Jul-Aug;25(4):327-8.
16. Jacobson DM. Symptomatic compression of the optic nerve by the carotid artery: clinical profile of 18 patients with 24 affected eyes identified by magnetic resonance imaging. Ophthalmology. 1999 Oct;106(10):1994-2004.
17. Greenfield DS, Siatkowski RM, Glaser JS, et al. The cupped disc: Who needs neuroimaging? Ophthalmology. 1998 Oct;105:1866-74.

