 Managing the dry eye patient has long been a regular component of the therapeutic optometric practice. Over the years, we’ve been fortunate to gain significant developments in treatments and preparations that allow us to manage our patients better. But there hasn’t been much innovation in diagnostic tools. We’ve been limited to longstanding but inexact measures such as Schirmer testing, phenol red thread testing, tear film break-up time measurement, and so forth. But lately, no truly objective, quantitative tests have appeared.
Managing the dry eye patient has long been a regular component of the therapeutic optometric practice. Over the years, we’ve been fortunate to gain significant developments in treatments and preparations that allow us to manage our patients better. But there hasn’t been much innovation in diagnostic tools. We’ve been limited to longstanding but inexact measures such as Schirmer testing, phenol red thread testing, tear film break-up time measurement, and so forth. But lately, no truly objective, quantitative tests have appeared.
Enter TearLab.
The TearLab Osmolarity System is a device and clinical lab test that is intended to measure the osmolarity of human tears to aid in the diagnosis of patients suspected of having dry eye disease, in conjunction with other methods of clinical evaluation. TearLab is for professional in vitro diagnostic use only. In fact, it may be one of the only objective tools we have to aid us in diagnosing and managing clinically relevant dry eye.
Also, it’s now reimbursable in most instances.
A Step in the Right Direction
Coding for the TearLab is not complex, but it is different because it’s considered to be a clinical lab test, not a typical office procedure. The TearLab Osmolarity System is an in vitro laboratory device and, as such, testing for Medicare patients is billed under the Clinical Diagnostic Laboratory Fee Schedule. Unlike the Physician Fee Schedule, Medicare patient co-payments or deductibles do not apply to services billed under the laboratory fee schedule—covered services are 100% reimbursed. Payment by the Centers for Medicare and Medicaid Services (CMS) is the lesser of: the amount billed; the local fee for a geographic area; or a national limit.
Under the laboratory fee schedule, CMS will only reimburse providers performing laboratory tests who maintain a current certificate as required by the Clinical Laboratory Improvement Amendments (CLIA). However, as of July 2, TearLab had its CLIA status waived, thus allowing those practitioners who have CLIA waiver certification to perform this test in their offices.
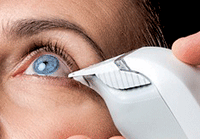
The electrical impedance osmometer takes an almost microscopic sample, just 50 nanoliters.
How do you obtain a CLIA waiver certificate? Submit the CMS-116 application form. Note that state laws in California, Nevada and New York don’t allow optometrists to obtain CLIA waiver certificates.
The CPT code to use is 83861 and is defined as: “Microfluidic analysis utilizing an integrated collection and analysis device, tear osmolarity.” Because it is a CLIA-waived test, you should use the modifier -QW (i.e., CLIA waived) when performing it in your office. Additionally, it is a unilateral test, so it requires an appropriate modifier if both eyes are tested on the same day. The modifiers for this are RT and LT (right and left).
So, the coding for the test looks like this if performed bilaterally:
- 83861-QW-RT
- 83861-QW-LT
The CMS reimbursement for this test nationwide is $23.40 per eye and there is no limit on the number of tests that you can run, as long as you have medical necessity established in the medical record.
Whether you believe in the clinical value of tear osmolarity or not, the development of an in-office test that allows us to diagnostically assess our patients and to evaluate our treatment protocols by objective measure is a step in the right direction.
Dr. Rumpakis has no financial affiliation with TearLab. Please send your comments to CodingAbstract@gmail.com.
1. TearLab Corp. website. Available at: www.tearlab.com/products/doctors/productinfo.htm. Accessed July 29, 2012.

