Management strategies in advanced cases include observation, topical and oral medications (such as oral NSAIDs or doxycycline), bandage contact lenses, epithelial debridement, anterior stromal puncture or therapeutic laser. Phototherapeutic keratectomy (PTK) in the treatment of EBMD and RCE can successfully eliminate the need for a more traumatic or invasive treatment plan.
Patient History
An established patient, a 52-year-old white female, presented for a routine examination in May 2006 with a chief complaint of blurred vision in her right eye that had become noticeable two weeks before her visit. She reported no double vision, loss of peripheral vision, change in pupil size, change in either lid position or pain; however, she did say that her vision loss appeared to be gradual.
She had been a compliant long-time disposable soft contact lens wearer for nearly 20 years without incident, and her ocular history was unremarkable. Likewise, her systemic history as well as her family’s ocular and systemic histories were also unremarkable.
Diagnostic Data
Best corrected visual acuity through -4.00 -0.50 x 090 O.D. and -5.50 -0.50 x 075 O.S. was 20/30 NIPH (no improvement with pinhole) and 20/20 respectively. Keratometry was unremarkable and unchanged compared to prior visits at 43.75 x 105 / 44.25 x 015 O.D. and 43.75 x 089 / 44.00 x 179 O.S. Humphrey Atlas topography with Pathfinder analysis revealed minimal corneal distortion in both eyes. Pupils were equal, round and responsive to light and accommodation. No afferent pupillary defect was present in the right eye.
Extraocular muscles were full with good alignment and without subjective diplopia. Ptosis was not evident in either eye. Red cap test was reported equal for both eyes. Humphrey threshold 120º visual fields were normal without evidence of defect in either eye. Biomicroscopic examination revealed significant corneal epithelial basement membrane disruption in each eye and greater in the right—it encroached upon the visual axis on that side.
There was no evidence of associated epithelial erosion, subepithelial microcystic edema, stromal edema or defect in either eye. Descemet’s folds were absent in both eyes. The endothelium was intact in both eyes. Grade IV angles were viewed in both eyes, and each demonstrated a deep and quiet anterior chamber. Her lenses were clear in both eyes. Dilated ophthalmoscopic evaluation of both eyes demonstrated unremarkable vitrea, peripheries, posterior poles and vessels. There was no evidence of maculopathy in either eye, and cup-to-disc ratios were 0.30 x 0.20 with healthy rim tissue.
Diagnosis
Based on the findings and bilateral presentation, I diagnosed the patient with epithelial basement membrane corneal dystrophy (O.S. > O.D.) with involvement of the paracentral visual axis O.D. and consequent reduction in visual acuity.
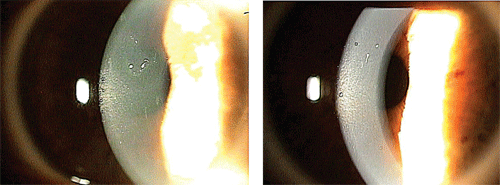
Note the central epithelial
basement membrane dystrophy (EBMD) in the visual axis of the right eye
(left). In the left eye, note the trace paracentral EBMD (right).
Treatment Strategy
Because of the increased incidence of secondary epithelial edema and erosion, she was placed on a treatment plan that utilizes frequent topical hyperosmotics:
• Muro 128 5% (sodium chloride hypertonicity ophthalmic oinment 5%, Bausch + Lomb) 1gtt q.i.d. O.U.
• Muro 128 5% ophthalmic ointment applied h.s. O.U.
Due to the chronic nature of the condition, involvement of the visual axis and decrease in visual acuity, phototherapeutic keratectomy was recommended for the right eye.
Considering the early basement membrane disruption present in the left eye, we gave the patient the option of bilateral PTK with additional simultaneous photorefractive keratectomy (PRK) O.U. to eliminate the refractive error. She had been a successful monovision soft contact lens wearer; so, a monovision treatment would be an ideal choice to consider.
She opted to schedule for dual PTK/PRK O.U., with an end goal of monovision. Taking into account her age and history of successful monovision contact lens wear with a dominant left eye, a target remaining refractive error of -1.50DS O.D. and plano O.S. was incorporated into the nomogram. We educated the patient about the limitations inherent to monovision correction regarding reduced binocularity and overall acuity. Additionally, per protocol, she completed and signed a surgical consent before receiving the treatment, which was scheduled for the following month.
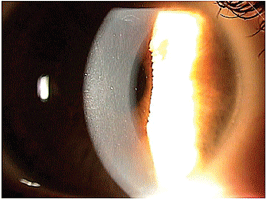
In the right eye after
treatment with PTK, note the clear central cornea with no evidence of
EBMD. But, trace EBMD can still be seen superiorly outside the
treatment zone.
Treatment
She returned to the practice for combined PTK/PRK in each eye, which proceeded without incident. An 8.6 +0.50D 14.2mm Proclear bandage contact lens was placed on each eye to allow for proper re-epithelialization and comfort. The lenses provided good coverage, centration and movement on each eye.
She was prescribed Vigamox (moxifloxacin 0.5%, Alcon) and Flarex (fluorometholone 0.1%, Alcon) 1gtt q.i.d. O.U. and scheduled for a one-day postoperative visit.
One-Day Follow-Up
She returned for her first follow-up visit reporting only a mild “scratchy feeling.” She already noticed a marked improvement in her vision both distance and near. She was compliant with her postoperative instructions.
Visual acuity through the bandage lenses was 20/J2 at 16” O.D., 20/25 O.S., and 20/25 O.U. Both her distance and near vision preliminarily represented the targeted monovision effect.
Biomicroscopic examination revealed a properly fitting bandage contact lenses over an expected large area of corneal epithelial defect in each eye. Neither cornea exhibited any indication of ensuing infiltrative keratopathy or edema. Additionally, the anterior chamber appeared clear in each eye with no evidence of surgically induced cell or flare.
We directed the patient to continue wearing the bandage contact lenses until full epitheliazation was complete—probably for an additional 48 to 72 hours. We also told her to continue her topical medications as instructed, and we scheduled a follow-up in three days.
Three-Day Follow-Up
She returned for her three-day follow-up and saw continued improvement in her vision and comfort. Acuity through the bandage contact lenses was stable at 20/J2 at 16” O.D., 20/25 O.S. and 20/25 O.U. distance and near.
Biomicroscopic evaluation of each eye revealed a characteristic central vertical seam of epithelium that signified a sealed, re-epithelialized corneal surface beneath the bandage lenses. There was no sign of subepithelial infiltrates or edema. The anterior chamber was clear and quiet in both eyes.
With corneal re-epithelialization complete, the contact lenses could be safely removed and the topical antibiotic Vigamox discontinued. But, we directed the patient to continue the Flarex 1gtt q.i.d. O.U. prophylactically against possible secondary inflammatory keratopathy. She was scheduled for a one-week follow-up visit.
One-Week Follow-Up
She returned as scheduled for her one-week visit reporting clear vision both far and near, as well as good comfort. Uncorrected VA was 20/J1 at 16” O.D., 20/20- O.S. and 20/20 O.U. at distance and near. Her refraction of -1.75DS O.D. at 20/20 and -0.25DS O.S. at 20/20 correlated nicely with the desired monovision result.
Biomicroscopic examination revealed only a trace remnant of the epithelial seam in each eye and no evidence of secondary keratopathy or anterior chamber reaction O.U. She was advised to slowly taper the Flarex over the next week and was scheduled to return in three weeks.
Three-Week Follow-Up
She returned for her three-week visit describing excellent vision and comfort. Uncorrected VA remained unchanged at 20/J1 at 16” O.D., 20/20- O.S. and 20/20 O.U. at distance and near—an optimal monovision outcome.
Biomicroscopic examination demonstrated a clear central cornea in each eye with no indication of disruption; however, trace dystrophy was visible outside the treatment zone, superior to the visual axis O.D.
Understanding the inherited etiology of EBMD, we discussed the possibility of recurrence to some degree or into the visual axis. Additionally, we spoke about the possibility of associated corneal edema and/or recurrent corneal erosion (RCE), as well as their respective symptoms.
I asked the patient to return in three months or as needed if she experienced a change in vision or developed discomfort.
Discussion
A dystrophy is essentially a genetically acquired condition that manifests itself later in life. Interestingly, the classic definition describes a dystrophy as a degenerative disorder caused by inadequate or defective nutrition.1 But, whether they arise from a congenital or nutritional basis, clinically, these conditions are typically progressive and have end-stage characteristics ranging from negligible to severe dysfunction, including vision loss or death.
Corneal dystrophies encompass a broad range of disorders that affect one or more of the corneal layers, including the epithelium, Bowman’s membrane, stroma, Descemet’s membrane and endothelium. As inherited conditions, they typically affect both eyes, although asymmetric disease progression may occur, which can create a more challenging initial presentation and diagnosis. Keratoconus, EBMD, granular corneal dystrophy, pre-Descemet’s membrane corneal dystrophy, and Fuchs’ endothelial corneal dystrophy are but a few of the corneal dystrophies known to exist.
The pathomorphology and pathophysiology of the corneal dystrophies is specific to the anatomic level of the disease process.
With respect to EBMD, a progressive pathology occurs, often after the forth decade of life, at the level of the epithelium and its corresponding basement membrane, producing an interruption of cellular integrity and tissue function.2
Resultant fluctuation or reduction in visual acuity, minimal to severe ocular discomfort or pain, epithelial edema, epithelial recurrent corneal erosion (RCE), or rarely, ruptured epithelial bullae may all be part of the clinical picture. However, many cases of EBMD can be observed clinically without the patient complaining about or even being aware of any signs or symptoms.
A number of treatment modalities are available, depending on the severity of the dystrophy, the development of RCE, the location with respect to the visual axis and ultimately, the effect on patient comfort or vision. Topical hyperosmotic solutions and ointments, epithelial debridement, bandage contact lenses, anterior stromal puncture, oral antibiotics and phototherapeutic keratectomy (PTK) are viable considerations.
In the case of EBMD resistant to traditional treatment, such as topical hyperosmotics or epithelial debridement, and if the EBMD is producing chronic visual disturbances or pain secondary to epithelial disruption, edema or erosion, PTK can be an ideal treatment option. The process is quick, precise, relatively painless and fast-healing.
Zones of epithelial and basement membrane involvement are often permanently eliminated with a high degree of success and alleviation of symptoms.
Additionally, the option of incorporating a refractive component into the procedure by adding photorefractive keratectomy (PRK) to the treatment plan allows for an even higher level of patient satisfaction.
A History of EBMD
Epithelial basement membrane corneal dystrophy, also known as Cogan microcystic epithelial corneal dystrophy or map-dot-fingerprint corneal dystrophy, is a common bilateral epithelial dystrophy with prevalence estimates ranging from 2% to 43% in the general U.S. population.3 Of patients with map-dot-fingerprint dystrophy, 10% to 33% have recurrent corneal erosions. As many as 50% of patients with RCE have map-dot-fingerprint dystrophy.3 EBMD has been found to be slightly more common in women than in men and is uncommon in children.3 The disorder may develop with little consequence or few symptoms, or it may ultimately create foreign body sensation, photophobia, pain, and/or visual obscurations that are very noticeable to the patient—as was the case with our patient.
In its earliest clinically apparent stages, the natural progression of the dystrophy can be quite unpredictable with respect to speed, direction and degree of keratopathy formation. During its course, keratometric and related refractive changes, including irregular astigmatism, may become more frequent. Viewed with wide-beam slit lamp biomicroscopy, corneal “maps” are irregular geographic shapes, or faint gray-white patches that may contain clear oval areas. They can vary in size from approximately 1mm to several millimeters in width. The “dots” are gray-white, putty-like opacities. They can be round, comma-shaped or irregular and are usually 0.05mm to 1mm in size. “Fingerprints” are clusters of contoured concentric lines between 0.25mm and 4mm long.3 More specifically, “maps” histologically represent areas of multilaminar basement membrane that extend into the epithelium. “Dots” are intraepithelial microcysts that contain nuclear, cytoplasmic and lipid debris. “Fingerprints” are curvilinear clusters of reduplicated and thickened basement membrane and fibrillogranular material. Blebs or epithelial bullae are a less common manifestation of map-dot-fingerprint dystrophy and are localized areas of fibrillogranular material or thickened basement membrane.3
The pathomorphology of EBMD has also been clinically distinguished by in vivo confocal microscopy using the HRT II Rostock Cornea Module (Heidelberg Engineering), which has confirmed distinctive microstructural characteristics. Particularly, abnormal basement membrane has been examined in this manner showing faulty anterior protrusions toward associated epithelium thereby contributing to poor adhesion and resultant pathology.4 This poor adhesion of epithelium and basement membrane allows for the distinctive expression of the disease process.
The most common secondary corneal sequelae evident on slit lamp biomicroscopy are microcystic edema and episodes of recurrent corneal erosion, which, of course, directly relate to patient symptoms and comfort. Frequently, symptoms are most dramatic in the morning hours upon awakening due to increased epithelial/subepithelial fluid retention, which results in edema or erosion. As exhibited in the case of our patient, central corneal involvement into the visual axis increases the likelihood of a consequent reduction or fluctuation in visual acuity.
The pathophysiology of EBMD leading to RCE can be best understood by considering the known mechanism in which corneal epithelium produces and normally tightly adheres to its underlying basement membrane. Faulty basement membrane production, which is thickened, multilaminar and misdirected into the epithelium, disrupts this normally congruous relationship. Deeper epithelial cells that normally migrate to the surface can become trapped. Epithelial cells anterior to the aberrant basement membrane may have difficulty forming viable hemidesmosomes and basement membrane complexes, which normally attach to the underlying stroma, ultimately resulting in recurrent erosions.3 An autosomal dominant pattern of inheritance has been reported.5 Two families revealing this pattern of inheritance, as well as an analysis of single affected individuals, have been identified with two different point mutations in the TGFBI/BIGH3 genes, which are known to be associated with other corneal dystrophies. This is the first report of a molecular mutation in individuals with EBMD and it increases the spectrum of mutations in the TGFBI/BIGH3 gene. Based on this study, up to 10% of EBMD patients could have a mutation in this gene.5
Treatment Strategies
Treatment considerations vary greatly depending on the extent of corneal involvement and patient symptoms. The main strategy is to educate and reassure the patient that most cases of basement membrane dystrophy are asymptomatic, and to reassure him or her that observation is the only necessary long-term approach.
In the event of progressive keratopathy with frequent RCE associated with pain or a reduction in VA, however, more aggressive medical or surgical treatment options may be indicated. Certainly, taking a stepped, methodical approach to treatment will be best tolerated by the patient, considering that initial non-invasive medical therapies are often successful.
First-line treatment with such hyperosmotic solutions and ointments as Muro 128 5% NaCl dosed 1gtt q.i.d. and ointment application before bedtime is an effective measure to reduce the likelihood of corneal edema and RCE. With recalcitrant cases of RCE, hyperosmotics can be prescribed indefinitely if effective and tolerated well by the patient.
Bandage contact lenses with prophylactic topical antibiotic therapy can be used effectively with acute erosions until re-epithelialization is accomplished.
Oral doxycycline and minocycline also have a stabilizing effect on the corneal epithelium and basement membrane by decreasing the amount of fatty acids and metal metalloproteinases (MMP-2 and MMP-9) found in the tear film.6 Fatty acids, along with MMP-2 and MMP-9, disrupt basement membrane formation and result in abnormal hemidesmosome formation and junctional complexes, which are important for securing the corneal epithelium to the underlying stroma.6
Mechanical corneal epithelial debridement is another method that has been shown advantageous in the management of anterior basement membrane dystrophy (ABMD), particularly with cases that involve the visual axis and create a decrease in vision. One study concluded that a five-year cumulative probability of recurrence of ABMD after mechanical debridement was favorable, at 44.7%.7 Likewise, debridement with diamond burr polishing of the basement membrane was shown to be an effective and safe treatment for decreased vision caused by ABMD over an average follow-up of 21.8 months. No reported recurrences of dystrophic basement membrane were discovered within the treatment zones during this time period.8
Anterior stromal puncture is yet another alternative shown to be valuable. Anterior stromal puncture with insulin needles or nd:YAG laser may enhance epithelial adhesion to the basement membrane by scar formation; success rates up to 80% have been reported in the treatment of recalcitrant RCE.9
Finally, as reported in the case, excimer laser phototherapeutic keratectomy can be a safe, precise and predictable treatment modality for symptomatic EBMD or RCE resistant to other therapies.10 With the rapid availability and technological advancements of excimer LASIK, it should continue to become a more accepted and trusted option in chronic symptomatic or visually obstructive presentations. The successful case presented resulted in corneal clarity and improvement in corrected vision, which is exemplary of results expected when proper preoperative conditions are present and surgical protocol is followed.
Provided any contraindications to excimer laser photoablation do not exist (e.g., recent herpes simplex keratitis, collagen-vascular or auto-immune disease), PRK can also be safely incorporated into the treatment plan and can only further enhance the outcome.
A 2007 study found that eyes with map-dot-fingerprint dystrophy can also have irregular corneal cylinder, producing fluctuation or reduction in visual acuity.11 The application of PTK in these cases has provided significant improvement in visual acuity; correction of irregular astigmatism can be confirmed by videokeratography.11
So, irregular astigmatism can be solely of epithelial origin, and it has been affirmed that, in some eyes, an abnormal corneal epithelium also creates optical aberrations.11 This possibility should be taken into account when, for example, wavefront-guided stromal photoablation procedures are being planned. With a reduction of aberrations secondary to EBMD via primary PTK, the inclusion of initial wavefront data into a subsequent refractive PRK treatment plan should be questioned and perhaps omitted.
Regarding EBMD’s role in creating RCE, PTK with low pulse energy and quantity can be an effective and minimally invasive treatment modality to achieve fast and durable epithelial closure, to prevent recurrent corneal erosions, and to increase visual acuity in most patients with central corneal involvement.12
PTK is an especially important treatment consideration for RCE unmanageable with other therapies. Long-term data suggest that most patients treated with PTK do not develop recurrences and that side effects are minimal.13
EBMD is a relatively common finding in daily practice, and it rarely requires more than routine observation and patient reassurance. But, when a patient is symptomatic, the chronic and unpredictable characteristics of EBMD can challenge your therapeutic strategy. So, adopt a methodical thought process to assess objective clinical signs vs. the degree and frequency of patient complaints.
Dr. Martinelli is in private practice in Charleroi, Pa.
1. Medline Medical Dictionary. Available at: www2.merriam-webster.com/cgi-bin/mwmednlm?book=Medical&va=dystrophy (Accessed June 2007).
2. Verma A. Corneal Erosion, Recurrent. eMedicine. www.emedicine.com/oph/topic113.htm (Accessed June 2007).
3. Verdier D. Dystrophy, Map-Dot-Fingerprint. eMedicine. Available at: www.emedicine.com/oph/topic95.htm (Accessed June 2007).
4. Labbe A, Nicola RD, Dupas B, et al. Epithelial basement membrane dystrophy: evaluation with the HRT II Rostock Cornea Module. Ophthalmology. 2006 Aug;113(8):1301-8.
5. Boutboul S, Black GC, Moore JE, et al. A subset of patients with epithelial basement membrane corneal dystrophy have mutations in TGFBI/BIGH3. Hum Mutat. 2006 Jun;27(6):553-7.
6. Miller M. Managing recurrent corneal erosions. Contact Lens Spectrum. 2006 Feb. Available at: www.clspectrum.com/article.aspx?article=12964 (Accessed June 2007).
7. Itty S, Hamilton SS, Baratz KH, et al. Outcomes of Epithelial Debridement for Anterior Basement Membrane Dystrophy. Am J Ophthalmol. 2007 Aug;144(2):217-21.
8. Tzelikis PF, Rapuano CJ, Hammersmith KM, et al. Diamond burr treatment of poor vision from anterior basement membrane dystrophy. Am J Ophthalmol. 2005 Aug;140(2):308-10.
9. Ramamurthi S, Rahman MQ, Dutton GN, Ramaesh K. Pathogenesis, clinical features and management of recurrent corneal erosions. Eye. 2006 Jun;20(6):635-44.
10. Murillo-Lopez F. Myopia, PRK. eMedicine. Available at: www.emedicine.com/oph/topic667.htm (Accessed June 2007).
11. Zalentein WN, Holopainen JM, Tervo TM. Phototherapeutic keratectomy for epithelial irregular astigmatism: an emphasis on map-dot-fingerprint degeneration. J Refract Surg. 2007 Jan;23(1):50-7.
12. Pogorelov P, Langenbucher A, Kruse F, Seitz B. Long-term results of phototherapeutic keratectomy for corneal map-dot-fingerprint dystrophy (Cogan-Guerry). Cornea. 2006 Aug;25(7):774-7.
13. Baryla J, Pan YI, Hodge WG. Long-term efficacy of phototherapeutic keratectomy on recurrent corneal erosion syndrome. Cornea. 2006 Dec;25(10):1150-2.

