 |
A 35-year-old Hispanic female presented to the ophthalmic emergency department with vision loss OD for one week. Visual acuities were 20/70 OD and 20/50 OS. Intraocular pressures, pupils and anterior segment findings were normal. The right fundus had preretinal and vitreous hemorrhages (VHs) centrally, intraretinal hemorrhages and neovascularization along the major arcades (Figure 1). The left had hemorrhages and intraretinal microvascular abnormalities without neovascularization. OCT was also employed (Figure 2).
The patient’s medical history was significant for type 1 diabetes. She reported uncontrolled blood glucose levels consistently measuring above 300mg/dL. She was diagnosed with a VH secondary to proliferative diabetic retinopathy (PDR) in the right eye.
Another patient, a 67-year-old Hispanic male, presented with blurred vision OD for five days accompanied by dark spots and “spider webs.” Visual acuities were 20/100 OD and 20/20 OS. There was no neovascularization anteriorly, but a diffuse VH was seen in the right eye. The left eye fundus exam was unremarkable. Due to the limited view of the right fundus, an ultrasound was conducted, revealing a posterior vitreous detachment (PVD) without a retinal break or detachment (Figure 3).
The patient was advised to sleep with his head elevated and discontinue any non-prescribed blood thinners. Frequent observation with repeat ultrasonography was suggested. At last follow-up, the patient’s vision had improved to 20/25, and no retinal neovascularization or break was visible. He was diagnosed with a hemorrhagic PVD and did not require further intervention.
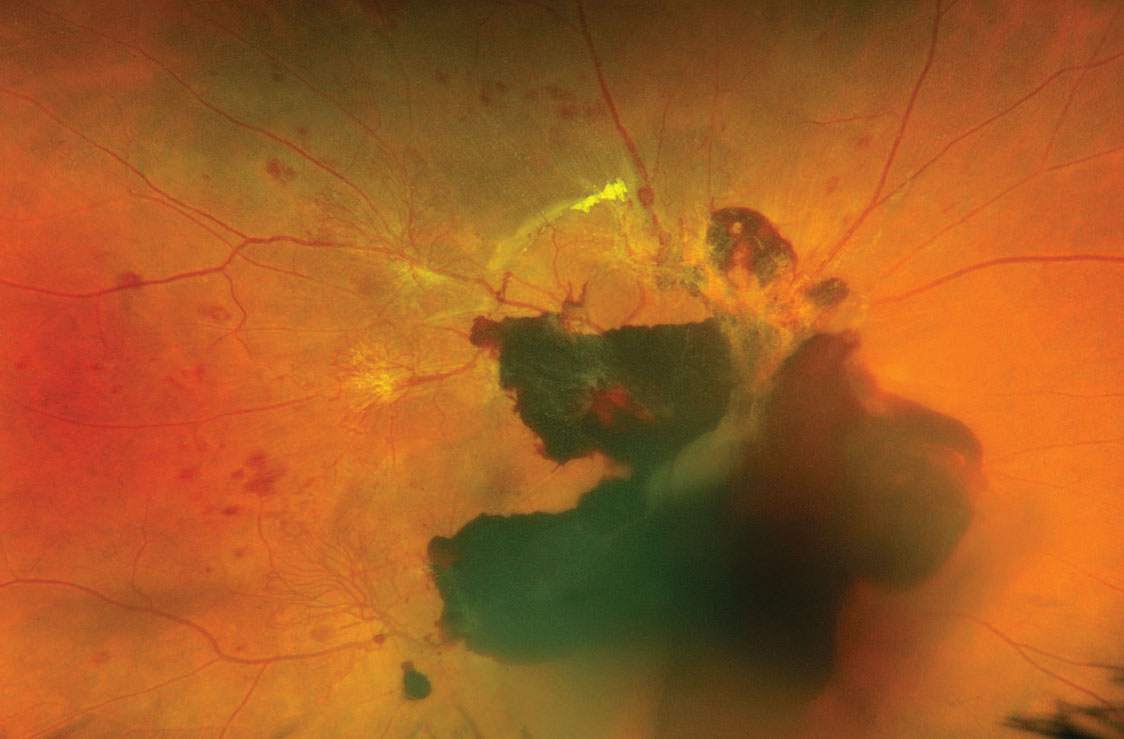 |
| Fig. 1. Fundus photo of the first patient shows vitreous and subhyaloid hemorrhages with active neovascular fronds extending from the arcades. Click image to enlarge. |
Etiology
These two patients have the same diagnosis, but their conditions are very different. VH can present for a multitude of reasons. Determining the best treatment underscores the importance of first reaching the correct diagnosis. Though exceptions exist, there are four main factors to consider when faced with a VH:
1. The most common etiology of a VH is neovascularization, and PDR remains the number one cause of all VHs.1 Intraocular neovascularization from ischemia can arise from numerous systemic conditions including diabetes, sickle cell anemia and leukemia.2,3 Ophthalmic conditions such as retinal vein or artery occlusions, ocular ischemic syndrome, chronic uveitis and chronic retinal detachment can also lead to neovascularization and VH.
2. The second cause of VHs is the rupture of a normal blood vessel. This can occur spontaneously or after ophthalmic trauma and is often seen in retinal tears, detachments and PVDs in part due to the strong vitreoretinal adhesions over retinal vessels.4 It is important to remember that eyes with a VH in the setting of an acute PVD have a 54% to 91% chance of a retinal break.5 Vessels on the optic nerve head are also susceptible to leakage when there is a shearing force or severe disc edema. More rarely, patients with Valsalva retinopathy or Terson’s syndrome may experience a hemorrhage that breaks into the vitreous cavity.
3. Third, consider the potential for an abnormal retinal vascular formation to rupture. Retinal arterial macroaneurysm lesions may lead to a VH.6,7 Clinical evidence of a lesion of this nature includes an extensive or multi-layered hemorrhage with or without a possible breakthrough VH. Rarer vascular causes of a VH are prepapillary loops and congenital retinal macrovessels.8,9
4. Finally, the choroid may be implicated in a VH. The retinal pigment epithelium (RPE) typically serves as a barrier between the choroidal circulation and the retina, but in cases of RPE breakdown, there remains potential for further complications. VHs have also been well-documented in conditions such as exudative age-related macular degeneration, choroidal melanoma and polypoidal choroidal vasculopathy.10-12
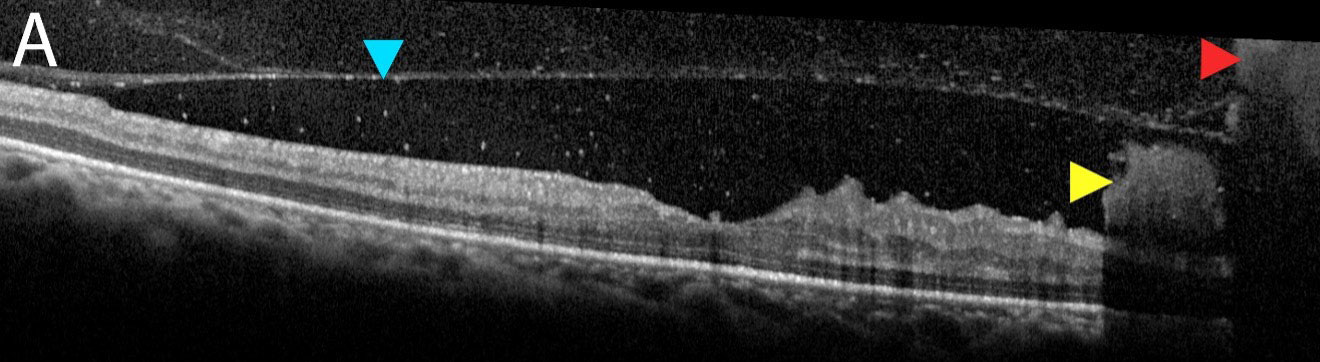 |
| Fig. 2a. OCT raster scan of the foveal pit demonstrates posterior hyaloid face (blue arrow) with both subhyaloid and preretinal hemorrhages (yellow arrow) and a VH (red arrow). Click image to enlarge. |
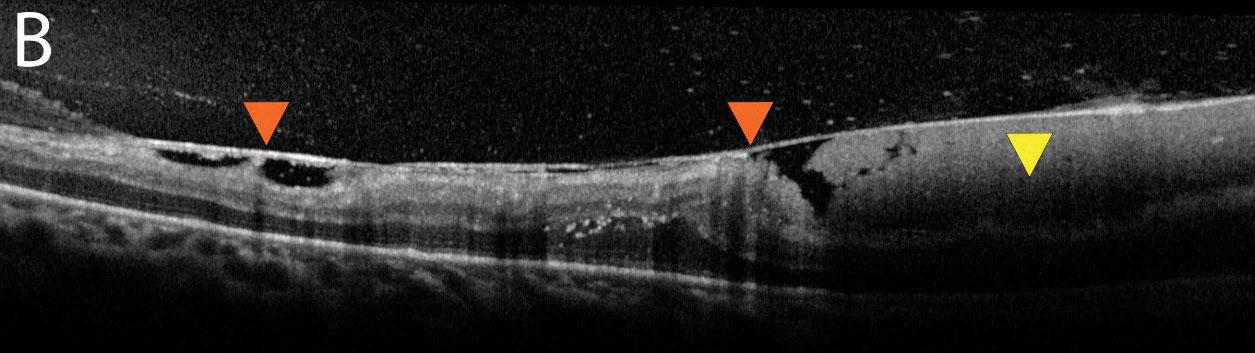 |
| Fig. 2b. An additional OCT image shows a thickened hyaloid with an adherent or “sticky” retina (orange arrows). The subhyaloid hemorrhage can be seen here in higher quality (yellow arrow). Click image to enlarge. |
Evaluation
When a patient with a VH presents, a review of systems should be completed. Knowledge of diabetes or a blood dyscrasia such as leukemia should raise suspicion of vascular compromise from ischemia. In cases where a systemic disease process is suspected, evaluation of the fellow eye is a crucial step.
In our first patient, the fellow eye examination supported diabetic retinopathy as a likely etiology. It is also beneficial to know the prior status of the affected eye. Inquire if the patient has had any previous ocular trauma, ophthalmic diagnoses, ocular surgeries, retinal laser procedures or intravitreal injections. A patient who was treated with intravitreal anti-VEGF agents for a retinal vein occlusion, for example, could be at an increased risk of intraocular neovascularization. Finally, symptoms such as photopsia or web-like floaters prior to vision loss could suggest an underlying PVD, retinal tear or retinal detachment.
Dynamic ultrasonography remains the gold standard for visualization of the vitreous cavity and retina in the case of a VH. In cases of ocular trauma, however, an open globe should first be ruled out given the risk of further globe compromise when pressure is applied. VHs often appear as mobile, poorly defined, low-level echoes.13 Attention should be directed to the retinal surface to evaluate for vitreoretinal traction, breaks or detachments. Additionally, ensure there are no choroidal masses present. Overall, diagnoses can be reliably made by those with experience in obtaining and reviewing imaging, but small retinal breaks can still be missed on ultrasonography, even by an experienced ultrasonographer or physician.14,15
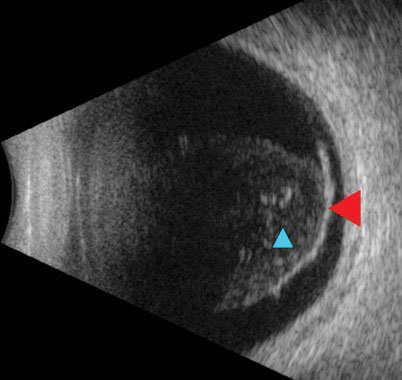 |
| Fig. 3. Ultrasound reveals a detachment of the posterior hyaloid face from the retina (red arrow). Notable vitreous debris (blue arrow) was appreciable on clinical exam as a hemorrhage. Click image to enlarge. |
Management
In general, the treatment of VHs is variable and dependent on the underlying condition. In cases of a VH from PDR, neither panretinal photocoagulation (PRP) nor intravitreal injection alone has been shown to improve the clearance rate of the hemorrhage, but PRP in isolation or in combination with anti-VEGF injections can prevent future VH episodes.16 Patients with neovascularization from PDR or retinal vein occlusions typically receive PRP to the ischemic retina once the VH clears enough to allow for visualization. If a retinal tear or detachment is visualized on ultrasonography, the patient should be referred to a retina surgeon emergently. Though there are proponents of early vitrectomy for a fundus-obscuring VH of any etiology, a decision may also be made to carefully monitor a patient with a VH as long as there is no ultrasonographic evidence of a retinal tear or etiology, as in the case of our second patient.5,17
A VH typically takes one to six months to clear, though they tend to resolve quicker in eyes that have undergone vitrectomy or syneresis. Non-clearing VHs are defined as those lasting longer than three months, and this is often an indication for vitrectomy regardless of etiology.
In all cases, a careful and systematic approach to evaluating VHs remains paramount. Referral and management strategy depends on the underlying pathology, so remain aware of the main etiologies behind this condition.
Dr. Bozung works in the Ophthalmic Emergency Department of the Bascom Palmer Eye Institute (BPEI) in Miami and serves as the clinical site director of the Optometric Student Externship Program as well as the associate director of the Optometric Residency Program at BPEI. She has no financial interests to disclose.
|
1. Spraul CW, Grossniklaus HE. Vitreous hemorrhage. Surv Ophthalmol, 1997;42(1):3-39. 2. Abdalla Elsayed MEA, Mura M, Al Dhibi H, et al. Sickle cell retinopathy. A focused review. Graefes Arch Clin Exp Ophthalmol, 2019;257(7):1353-64. 3. Soman S, Kasturi N, Srinivasan R, Vinod KV. Ocular manifestations in leukemias and their correlation with hematologic parameters at a tertiary care setting in South India. Ophthalmol Retina. 2018;2(1):17-23. 4. Gupta MP, Herzlich AA, Sauer T, Chan CC. Retinal anatomy and pathology. Dev Ophthalmol. 2016;55:7-17. 5. Melamud A, Pham H, Stoumbos Z. Early vitrectomy for spontaneous, fundus-obscuring vitreous hemorrhage. Am J Ophthalmol. 2015;160(5):1073-7. 6. Nakamura H, HayakawaK, Sawaguchi S, et al. Visual outcome after vitreous, sub-internal limiting membrane, and/or submacular hemorrhage removal associated with ruptured retinal arterial macroaneurysms. Graefes Arch Clin Exp Ophthalmol. 2008;246(5):661-9. 7. Pitkänen L, Tommila P, Kaarniranta K, et al. Retinal arterial macroaneurysms. Acta Ophthalmol. 2014;92(2):101-4. 8. Mansour AM, Kozak I, Saatci AO, et al. Prepapillary vascular loop-a new classification. Eye (Lond). 2021;35(2):425-32. 9. Goel N, Kumar V, Ghosh B. Congenital retinal macrovessel associated with vitreous hemorrhage. J AAPOS. 2017;21(1):83-5. 10. Kim TY, Kang HG, Choi EY, et al. Prognostic factors and long-term surgical outcomes for exudative age-related macular degeneration with breakthrough vitreous hemorrhage. Korean J Ophthalmol. 2020;34(4):281-9. 11. Fraser DJ Jr., Font RL. Ocular inflammation and hemorrhage as initial manifestations of uveal malignant melanoma. Incidence and prognosis. Arch Ophthalmol. 1979;97(7):1311-4. 12. Zhao XY, Luo MY, Meng LH, et al. The incidence, characteristics, management, prognosis, and classification of breakthrough vitreous hemorrhage secondary to polypoidal choroidal vasculopathy. Retina. 2021;41(8):1675-85. 13. Lorente-Ramos RM, Armán JA, Muñoz-Hernández A, et al. US of the eye made easy: a comprehensive how-to review with ophthalmoscopic correlation. Radiographics. 2012;32(5):175-200. 14. Parchand S, Singh R, Bhalekar S. Reliability of ocular ultrasonography findings for pre-surgical evaluation in various vitreo-retinal disorders. Semin Ophthalmol. 2014;29(4):236-41. 15. Rabinowitz R, Yagev R, Shoham A, Lifshitz T. Comparison between clinical and ultrasound findings in patients with vitreous hemorrhage. Eye (Lond). 2004;18(3):253-6. 16. El Annan J, Carvounis PE. Current management of vitreous hemorrhage due to proliferative diabetic retinopathy. Int Ophthalmol Clin. 2014;54(2):141-53. 17. Zhang T, Zhang J, Sun X, et al. Early vitrectomy for dense vitreous hemorrhage in adults with non-traumatic and non-diabetic retinopathy. J Int Med Res. 2017;45(6):2065-71. |

