Practical Matters in Myopia ManagementWith an expanding arsenal of information, myopia can be a puzzling area of care. In this second annual Review of Optometry supplement, experts offer solutions backed by science to help you manage this growing patient population. Click here to download a PDF. Check out the other articles featured in this supplement: |
All optometrists have familiarity with atropine as a diagnostic mainstay in routine dilation, as well as its therapeutic uses in managing iritis and other pupillary concerns. Somewhat newer, however, is the drug’s role in myopia management. Various low dosages of this topical nonselective muscarinic antagonist have been studied, and are routinely used, to curtail axial elongation through a presumed dopaminergic mechanism. This application of atropine is currently an off-label use in the United States.
Higher concentrations of atropine, ranging from 0.5% to 1%, have been used for years, but more recently, lower concentrations have demonstrated approximately 50% slowing of myopia progression.1-5
The ease of use as a once-nightly eye drop has made it a common treatment option for eye care practitioners to prescribe. A 2018 survey showed 70% of pediatric ophthalmologists are prescribing eye drops to decrease myopia progression, although only 8% of myopia control treatments prescribed by optometrists worldwide include pharmaceuticals.6,26 Despite its widespread use, there are still several unknowns when prescribing and obtaining low-dose atropine.
Which Concentration is Appropriate?
The story of atropine use for slowing myopia progression begins with the Atropine Treatment Of Myopia (ATOM) studies. In 2006, ATOM1 demonstrated that nightly monocular treatment with 1% atropine was effective in slowing axial elongation and myopia progression over two years compared to placebo (0.38mm, 1.20D vs. 0.02mm, 0.28D).1 Children aged six to 12 were able to tolerate the treatment and achieved myopia control effects. However, the researchers acknowledged the unwanted side effects of glare, photophobia and blurred near vision from cycloplegia. These were more tolerable in this study since the treatment was administered monocularly, but with binocular treatment these effects would become more deleterious.
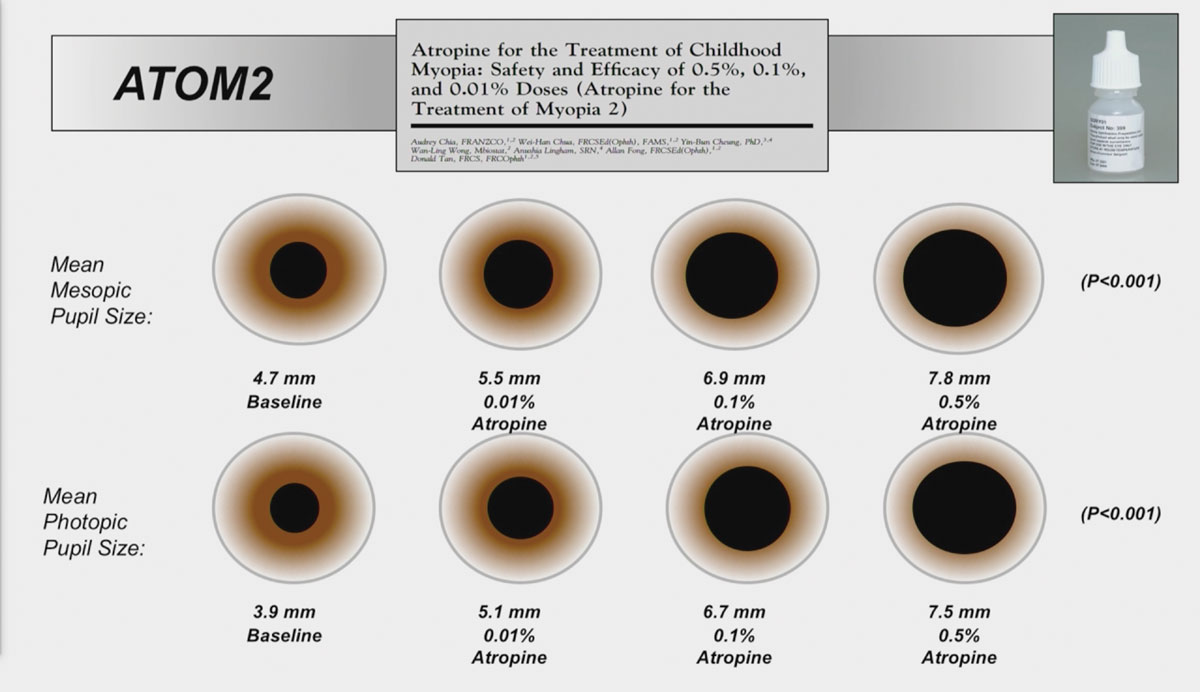
To mitigate the unwanted side effects, ATOM2 explored the nightly binocular treatment of lower concentrations of atropine (0.5%, 0.1%, or 0.01%) for two years.5 Historical data from ATOM1 was used as the control since it was considered unethical to withhold treatment after showing its efficacy. After two years, the researchers found that axial elongation and myopia progression were greater with lower concentrations (0.27mm, 0.30D with 0.5%; 0.28mm, 0.38D with 0.1%; and 0.41mm, 0.49D with 0.01%).
After two years, the medication was stopped for one year, then any children demonstrating myopia progression of 0.50D or more in at least one eye were restarted on 0.01% atropine.7 During the one year of cessation, there was a rebound effect, with an inverse dose-related increase in myopia with higher concentrations. After three years, more children in the higher concentration groups had to restart treatment due to 0.50D or more of progression (24% on 0.01%, 59% on 0.1% and 68% on 0.5%). The researchers concluded that, over five years, the 0.01% atropine was effective in slowing myopia progression with limited side effects and rebound. However, it was later pointed out that while atropine 0.01% was effective in slowing myopia progression, it had a limited effect on slowing axial elongation.8
With the recognition that perhaps lower concentrations are more tolerable while still maintaining efficacy, the Low-Concentration Atropine for Myopia Progression (LAMP) study was initiated in Hong Kong.9 The first phase of the study evaluated atropine 0.05%, 0.025%, 0.01% and placebo over one year in myopic Chinese children aged four to 12 years. As expected, the use of higher concentrations resulted in less axial elongation and myopia progression (0.20mm, 0.27D on 0.05%; 0.29mm, 0.46D on 0.025%; 0.36mm, 0.59D on 0.01%; and 0.41mm, 0.81D on placebo). In the second phase of the study, the children in the placebo group were switched to 0.05% atropine and the others continued with the same therapy.4 Those that switched demonstrated the efficacy of initiating treatment even after a year of placebo. In the final phase of the study, children in each group were randomized to either continue treatment or washout.10 Those that washed out showed a rebound effect, greater with higher concentrations, and those that continued treatment showed continued efficacy of the assigned therapy. The LAMP study results advocated using atropine 0.05% as the optimal concentration to achieve myopia control efficacy with limited side effects.
Based on the ATOM1, ATOM2 and LAMP studies, the primary concentration of first choice has been atropine 0.05% for many practitioners.
The ideal atropine concentration is effective in slowing both myopia progression and axial elongation while also mitigating the side effects of the drug. Systemic side effects can occur with higher concentrations, but with low-dose atropine the most common side effects are ocular. Glare, photophobia and rarely blurred near vision are the most common side effects. The LAMP study demonstrated that atropine 0.05% is similar in efficacy to optical treatments such as orthokeratology and soft multifocal contact lenses.11 However, the debate on the optimal concentration of atropine is still undecided and may differ by patient.
The complexity of the concentration debate increased as two additional clinical studies were published in 2023. The Child Atropine for Myopia Progression (CHAMP) study compared preservative-free low-dose atropine in two concentrations, 0.01% and 0.02%, and was the first placebo-controlled study in the US and Europe to compare the safety and efficacy of low-dose atropine use in children as a treatment for myopia.12 Participants from ages three to 17 were included, with varying levels of myopia (-0.50 to -6.00 D). The 0.01% concentration slowed axial elongation and myopia progression more than the 0.02% concentration (least squares mean difference: 0.13mm, 0.24D vs. 0.08mm, 0.10D). These results raised questions about clinical significance vs. statistical efficacy over three years of treatment, and whether 0.13mm axial length and 0.24D Rx reductions (vs. placebo) justify a decision to treat.
The FDA also required the study to add responder analysis as an endpoint, or how many children progressed -0.50D or less over the three-year study. Thirty percent of those using 0.01% atropine met this endpoint, which was statistically significant. The study also demonstrated the safety of the medication over the three years, with no reported serious ocular or systemic adverse events.
In contrast, the Pediatric Eye Disease Investigator Group (PEDIG) completed a 30-month study in US children aged five to 12 years that compared atropine 0.01% to placebo.13 After two years of nightly bilateral instillation, there was no difference between the atropine 0.01% group and the placebo group in terms of axial length or spherical equivalent refractive error change from baseline (0.44mm, -0.82D vs. 0.45mm, -0.80D).
The lack of treatment effect is not likely due to compliance or participant retention, but the results conflict with previous studies showing the efficacy of atropine 0.01% against placebo.
The authors point out that most of these studies demonstrating a treatment effect were done in East Asian and South Asian populations, whereas this study was performed in the US with almost half of the sample being Caucasian children. Walline and Berntsen point out that the discrepancy in findings between Asian and Caucasian children may be a consequence of differences in iris pigmentation, study length, age and rate of myopia progression.14 Interestingly, they note that darker irises, more common amongst Asian children, contain more melanin, which atropine binds to and may continue to release for a longer period.
Regardless, the CHAMP study results offer hope that there may be an FDA-approved low-dose atropine compound available in the future. An approved marketed product would resolve many issues that arise with the use of compounded products—including stability, sterility and efficacy. There would be greater assurance that the inconsistencies between the product and its storage or use would affect the delivery of the medication. Practitioners could feel more confident in prescribing a medication that is true to the intended concentration, and parents may be less apprehensive about sterility issues that could pose safety concerns.
 |
Treatment Initiation, Duration and Cessation
Though the mechanism of atropine in slowing myopia progression is still unknown, it is clear that it can be an effective therapy for children. As with all myopia control therapies, it is best to initiate treatment as soon as possible. The benefit of atropine is the ease of use, specifically in younger children who may not be ready for contact lens wear. Low-dose atropine has been shown to be effective in children as young as three years old.12
During low-dose atropine use, it is important to monitor for side effects, specifically photophobia and near blur. While children are very adaptable and tolerant, these potential side effects could affect their visual performance, academic achievement and quality of life. If these side effects are deemed intolerable, practitioners can consider prescribing light-adaptive bifocal spectacle lenses, altering the time of administration or decreasing the atropine concentration.
With its ease of use, low-dose atropine is also easily added to monotherapy of orthokeratology or soft multifocal contact lens use. With different mechanisms of action, it is assumed that combining atropine and contact lenses would further slow myopia progression. Several randomized control trials have shown that there is an additive effect of atropine 0.01% with orthokeratology lens wear than orthokeratology alone.15,16 Most recently, one retrospective study found that this additive effect was only significant for the first 1.5 years.17 Over the course of the two-year study, there was a significantly smaller change in axial length with combination therapy compared to orthokeratology alone for the first three six-month periods, but there was no difference in axial elongation between the two groups during the last six-month period (0.05mm, 0.04mm, 0.05mm vs. 0.03mm). Additionally, the study analyzed age at baseline and found that the difference in combination therapy vs. orthokeratology alone in children 10 years or younger could achieve a greater effect of slowing axial elongation (0.24mm vs. 0.07mm in children over 10 years).
A recent study comparing soft multifocal contact lenses and 0.01% atropine to soft multifocal contact lenses alone did not find a statistically significant slowing of eye growth, although the difference between the combination and monotherapies was similar in magnitude to the differences using 0.01% atropine and orthokeratology.18 The combination therapy group showed similar slowing of axial elongation and myopia progression as the soft multifocal lens group alone (0.31mm, 0.52D vs. 0.39mm, 0.55D) over a three-year period.
Parents are always concerned with how long their child must be on these therapies, perhaps even more so when medication is involved. The World Health Organization recommends only two years of use; however, several studies have shown efficacy over longer periods of time.19 There are also no reported long-term side effects of low-dose atropine use.20 With such low concentrations of atropine, the safety profile is likely greater than that of commercially available 1% atropine, often used for penalization in amblyopia therapy. In all studies observing the potential for a rebound effect, the medication was discontinued and not gradually decreased in frequency or concentration. Ultimately, low-dose atropine can be a safe and effective treatment for slowing myopia progression in young children as a monotherapy or combination therapy.
 |
Inconsistencies in Compounding
Presently, low-dose atropine must be obtained directly from a compounding pharmacy. These outlets are not held to the same strict guidelines by the FDA as commercial drug manufacturers. Rather, they operate under the jurisdiction of their state boards of pharmacy and are not required to undergo the same rigorous product testing. This discrepancy between manufacturing facilities may affect the safety and efficacy of these low-dose atropine products.
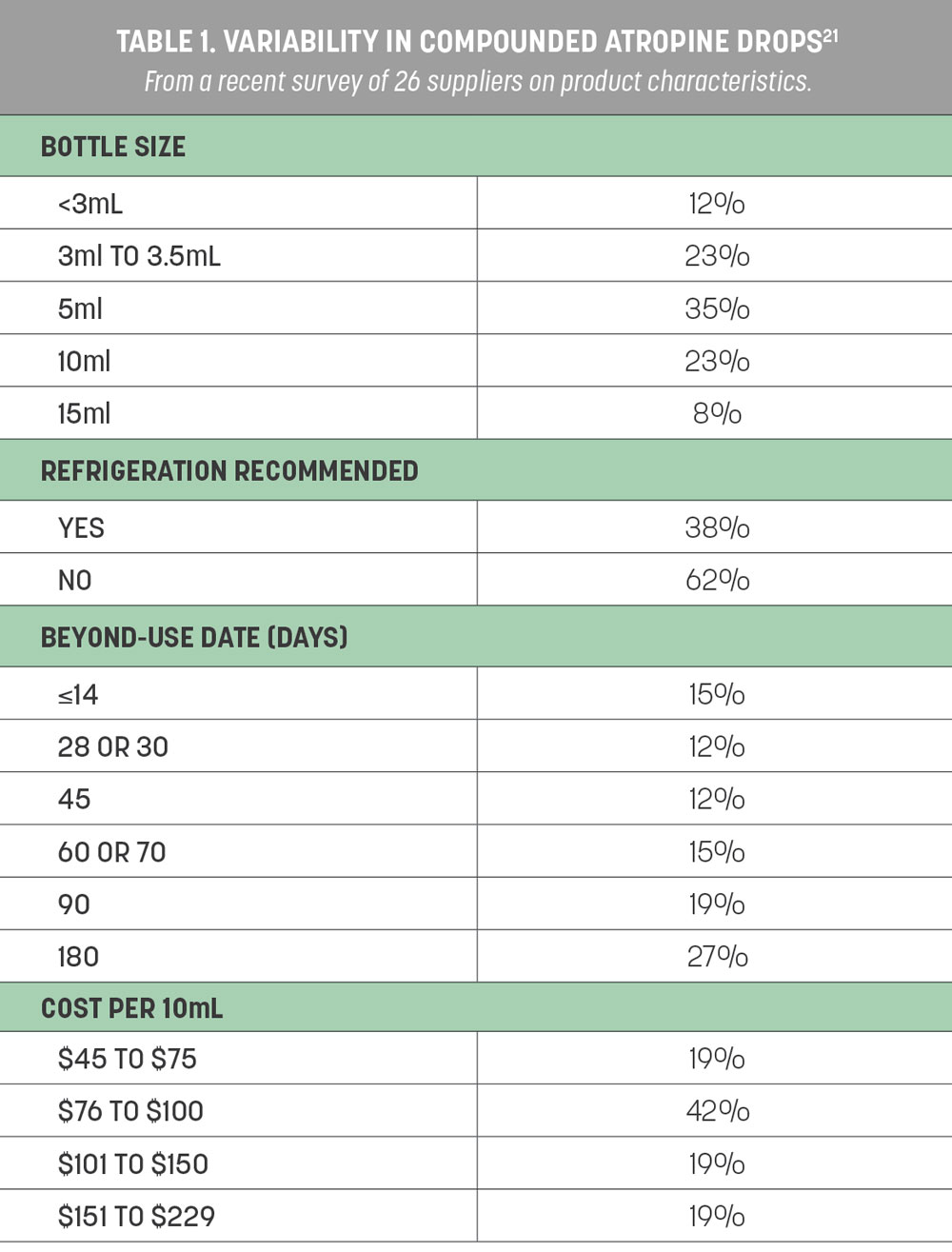
A recent survey of 26 different compounding pharmacies across 19 states was conducted to find out more about the compounding and manufacturing of low-dose atropine.21 The pharmacies were asked specific questions about their products, including storage instructions, bottle size, beyond-use dates and compounding ingredients (Table 1). These factors may affect the quality, stability and sterility of compounded low-dose atropine products.
The average reported bottle size was a 5mL bottle, which should contain about 100 drops and last about 50 days with nightly bilateral use. The median beyond-use date was 65 days (range: 45-158); however, some pharmacies specified a shorter time frame once the bottle was opened (e.g., 28 or 30 days). Some pharmacies prepare their formulations in batches, so the beyond-use date may not be from the date of receipt or opening. Many of the smaller pharmacies prepared the medication to order. The beyond-use dates indicate how long the product can maintain its stability and potency against degradation after it is made.
The active ingredient may be sourced from US Pharmacopeia (USP) powdered atropine or commercially available 1% atropine ophthalmic solution. Inactive ingredients are added to dilute the concentration or increase sterility, tolerability and efficacy. Half of the pharmacies reported using commercially available 1% atropine solution as the active ingredient, whereas 38% used USP powdered atropine. Commercially available artificial tears were mostly commonly used for dilution (42%), followed by saline (23%). The remainder used more than one inactive ingredient, including preservatives like benzalkonium chloride (BAK) for sterility or boric acid to lower the pH. BAK is found in commercially available 1% atropine solution, so if this is used as the base for low-dose concentrations, these preservatives are likely diluted by other inactive ingredients, resulting in a low level of preservatives that may be ineffective in maintaining a product’s sterility.
Most smaller compounding pharmacies do not have the capability to do analytical testing of their products routinely. Some may use analytical methods on batches of drugs being compounded, but it is unlikely that testing of stability over time is completed. Conversely, FDA-approved marketed products must undergo extensive testing for sterility, efficacy and stability until the expiration date. Atropine in solution is intrinsically unstable and breaks down into tropic acid and tropine.22 Both pH and storage temperatures will affect the stability of these low-dose products and their potency over time. The limited testing may also pose a public health risk as the contamination of products and subsequent widespread use can occur. Several outbreaks with ocular medications have resulted in permanent vision loss from contaminated compounded drugs.23,24
It is imperative that any prescribed drug, including low-dose atropine, is a quality product that will remain sterile and stable for the duration of its use. With compounded medications, variable formulations and a lack of consistent analytical testing, it becomes more challenging to ensure repeatability between products for patients.
To explore potential product inconsistencies, the same researchers obtained 24 samples of 0.01% atropine from nine different compounding pharmacies.25 They sent the samples to be analyzed 30 days after receipt and found a wide variety of formulations. The median pH of the samples was 6.9, close to that of the ocular surface. Atropine is stable at lower pH levels (2-4), but most ophthalmic solutions are closer to neutral (6.6-7.8). At higher pH levels, atropine degrades faster to tropic acid, which has no antimuscarinic properties. Samples from two pharmacies had tropic acid concentrations greater than the USP limit, indicating the breakdown of atropine. Though lower pH levels may cause stinging or discomfort for the patient upon instillation, the lower pH increases the drug stability.
Thirty days after receipt, the median percent concentration of atropine was 7% less than the target (i.e., 93%), with six samples having less than 90% of the target. The concentration should be within 10% of the prescribed or labeled concentration. Given that 0.01% is the lowest concentration of atropine used clinically, this poses concerns for efficacy. Research is still developing regarding the tolerable or most effective concentration, but perhaps results can be skewed if the concentration eye care providers are prescribing is not truly what the patient is receiving.
 |
|
Fig. 3. Assess the retina with a dilated fundus examination for any current myopic changes and set a baseline for future comparisons. Click image to enlarge. |
Greater Certainty Needed
The aforementioned issues with the stability of low-dose atropine can lead to inconsistent treatment results for patients. Variable quality of products can be a public health risk. Furthermore, the ongoing uncertainty over which dose is optimal for specific patient populations adds a level of guesswork that clinicians would clearly prefer to do away with. With increased prescribing of low-dose atropine for myopia management, there is a need for a commercial product that can be held to higher standards of regulation. With the CHAMP study results, it is clear that an FDA-approved product may be available in the near future.12 The debate about which concentration of low-dose atropine is most effective will continue as new research emerges.
Dr. Tomiyama is an assistant professor of optometry at Marshall B. Ketchum University, where she offers a contact lens curriculum and serves as a clinical attending in the Stein Family Cornea & Contact Lens Center. She recently established the Myopia Management Service at the university. She receives consulting fees from Vyluma and conducts research with CooperVision.
1. Chua WH, Balakrishnan V, Chan YH, et al. Atropine for the Treatment of Childhood Myopia. Ophthalmology. 2006;113(12):2285-2291. 2. Chou AC, Shih YF, Ho TC, Lin LL. The Effectiveness of 0.5% Atropine in Controlling High Myopia in Children. Journal of Ocular Pharmacology and Therapeutics. 1997;13(1):61-67. 3. Shih YF, Chen CH, Chou AC, Ho TC, Lin LL, Hung PT. Effects of Different Concentrations of Atropine on Controlling Myopia in Myopic Children. Journal of Ocular Pharmacology and Therapeutics. 1999;15(1):85-90. 4. Yam JC, Li FF, Zhang X, et al. Two-Year Clinical Trial of the Low-Concentration Atropine for Myopia Progression (LAMP) Study: Phase 2 Report. Ophthalmology. 2020;127(7):910-919. 5. Chia A, Chua WH, Cheung YB, et al. Atropine for the Treatment of Childhood Myopia: Safety and Efficacy of 0.5%, 0.1%, and 0.01% Doses (Atropine for the Treatment of Myopia 2). Ophthalmology. 2012;119(2):347-354. 6. Zloto O, Wygnanski-Jaffe T, Farzavandi SK, Gomez-de-Liaño R, Sprunger DT, Mezer E. Current trends among pediatric ophthalmologists to decrease myopia progression—an international perspective. Graefe’s Archive for Clinical and Experimental Ophthalmology. 2018;256(12):2457-2466. 7. Chia A, Lu QS, Tan D. Five-Year Clinical Trial on Atropine for the Treatment of Myopia 2 Myopia Control with Atropine 0.01% Eyedrops. Ophthalmology. 2016;123(2):391-399. 8. Bullimore MA, Berntsen DA. Low-dose atropine for myopia control: Considering all the data. JAMA Ophthalmol. 2018;136(3):303. 9. Yam JC, Jiang Y, Tang SM, et al. Low-Concentration Atropine for Myopia Progression (LAMP) Study. Ophthalmology. 2019;126(1):113-124. 10. Yam JC, Zhang XJ, Zhang Y, et al. Three-Year Clinical Trial of Low-concentration Atropine for Myopia Progression (LAMP) Study: Continued Versus Washout. Phase 3 Report. Ophthalmology. 2021;129(3):308-321. 11. Huang J, Wen D, Wang Q, et al. Efficacy Comparison of 16 Interventions for Myopia Control in Children. Ophthalmology. 2016;123(4):697-708. 12. Zadnik K, Schulman E, Flitcroft I, et al. Efficacy and Safety of 0.01% and 0.02% Atropine for the Treatment of Pediatric Myopia Progression Over 3 Years. JAMA Ophthalmol. 2023;137(4):408-414. 13. Repka MX, Weise KK, Chandler DL, et al. Low-Dose 0.01% Atropine Eye Drops vs Placebo for Myopia Control: A Randomized Clinical Trial. JAMA Ophthalmol. Published online 2023:1-11. 14. Walline JJ, Berntsen DA. Atropine, 0.01%, for Myopia Control. JAMA Ophthalmol. 2023;50(9):1001-1012. 15. Kinoshita N, Konno Y, Hamada N, et al. Efficacy of combined orthokeratology and 0.01% atropine solution for slowing axial elongation in children with myopia: a 2-year randomised trial. Sci Rep. 2020;10(1):1-11. 16. Tan Q, Ng ALK, Choy BNK, Cheng GPM, Woo VCP, Cho P. One-year results of 0.01% atropine with orthokeratology (AOK) study: a randomised clinical trial. Ophthalmic and Physiological Optics. 2020;40:557-566. 17. Du L, Chen J, Ding L, et al. Add-On Effect of 0.01% Atropine in Orthokeratology Wearers for Myopia Control in Children: A 2-Year Retrospective Study. Ophthalmol Ther. Published online 2023. 18. Jones JH, Mutti DO, Jones-Jordan LA, Walline JJ. Effect of Combining 0.01% Atropine with Soft Multifocal Contact Lenses on Myopia Progression in Children. Optometry and Vision Science. 2022;99(5):434-442. 19. Verhoeven VJM, Wong KT, Buitendijk GHS, Hofman A, Vingerling JR, Klaver CCW. Visual Consequences of Refractive Errors in the General Population. Ophthalmology. 2015;122(1):101-109. 20. Bullimore MA, Ritchey ER, Shah S, Leveziel N, Bourne RRA, Flitcroft DI. The Risks and Benefits of Myopia Control. Ophthalmology. 2021;128(11):1561-1579. 21. Richdale K, Tomiyama ES, Novack GD, Bullimore MA. Compounding of Low-Concentration Atropine for Myopia Control. Eye & Contact Lens: Science & Clinical Practice. 2022;48(12):489-492. 22. Zvirblis P, Socholitsky I, Kondritzer AA. The Kinetics of the Hydrolysis of Atropine. Journal of the American Pharmaceutical Association (Scientific ed). 1956;45(7):450-454. 23. Sheyman AT, Cohen BZ, Friedman AH, Ackert JM. An Outbreak of Fungal Endophthalmitis After Intravitreal Injection of Compounded Combined Bevacizumab and Triamcinolone. JAMA Ophthalmol. 2013;131(7):864. 24. Gonzalez S, Rosenfeld PJ, Stewart MW, Brown J, Murphy SP. Avastin Doesn’t Blind People, People Blind People. Am J Ophthalmol. 2012;153(2):196-203.e1. 25. Richdale K, Skidmore K V., Tomiyama ES, Bullimore MA. Compounded 0.01% Atropine-What’s in the Bottle? Eye Contact Lens. 2023;49(6):219-223. 26. Wolffsohn JS, Calossi A, Cho P, Gifford K, Jones L, Jones D, et al. Global trends in myopia management attitudes and strategies in clinical practice – 2019 Update. Contact Lens and Anterior Eye. 2020;43(1):9-17. |

