 |
A 57-year-old Peruvian female presented with painless decrease in vision OD for one month. She reported a similar decline in vision in the same eye one year prior that was treated with intravitreal injections. Past medical, social and family histories were unremarkable. VA was 20/100 OD and 20/20 OS with no pinhole improvement. Pupils were equally round and reactive without a relative afferent pupillary defect, confrontation visual fields were full OU and extraocular motilities were full OU. IOP was 13mm Hg OD and 12mm Hg OS. Slit lamp exam was unremarkable.
Take the Retina Quiz
1. This patient’s condition is most likely related to which organism?
a. Borrelia burgdorferi.
b. Histoplasma capsulatum.
c. Toxocara canis.
d. Toxoplasma gondii.
2. Which of the following findings would NOT be consistent with this patient’s diagnosis?
a. Macular neovascularization (MNV).
b. Midperipheral or peripheral atrophic chorioretinal lesions.
c. Peripapillary atrophy.
d. Vitritis.
3. The causative organism is endemic to which of the following places?
a. Central America.
b. South America.
c. United States.
d. All of the above.
4. What is the most appropriate treatment for the acute management of this patient?
a. Intravitreal anti-vascular endothelial growth factor (VEGF).
b. Intravitreal triamcinolone and oral prednisone.
c. Laser photocoagulation.
d. Photodynamic therapy.
5. Which of the following is true?
a. Aggressive oral prednisone is necessary during the acute phase to minimize ocular sequelae.
b. Moderate systemic signs and symptoms requires systemic treatment.
c. Patients may contract this condition from soil contaminated by canine feces.
d. Vision loss is primarily due to MNV, not direct chorioretinal inflammation.
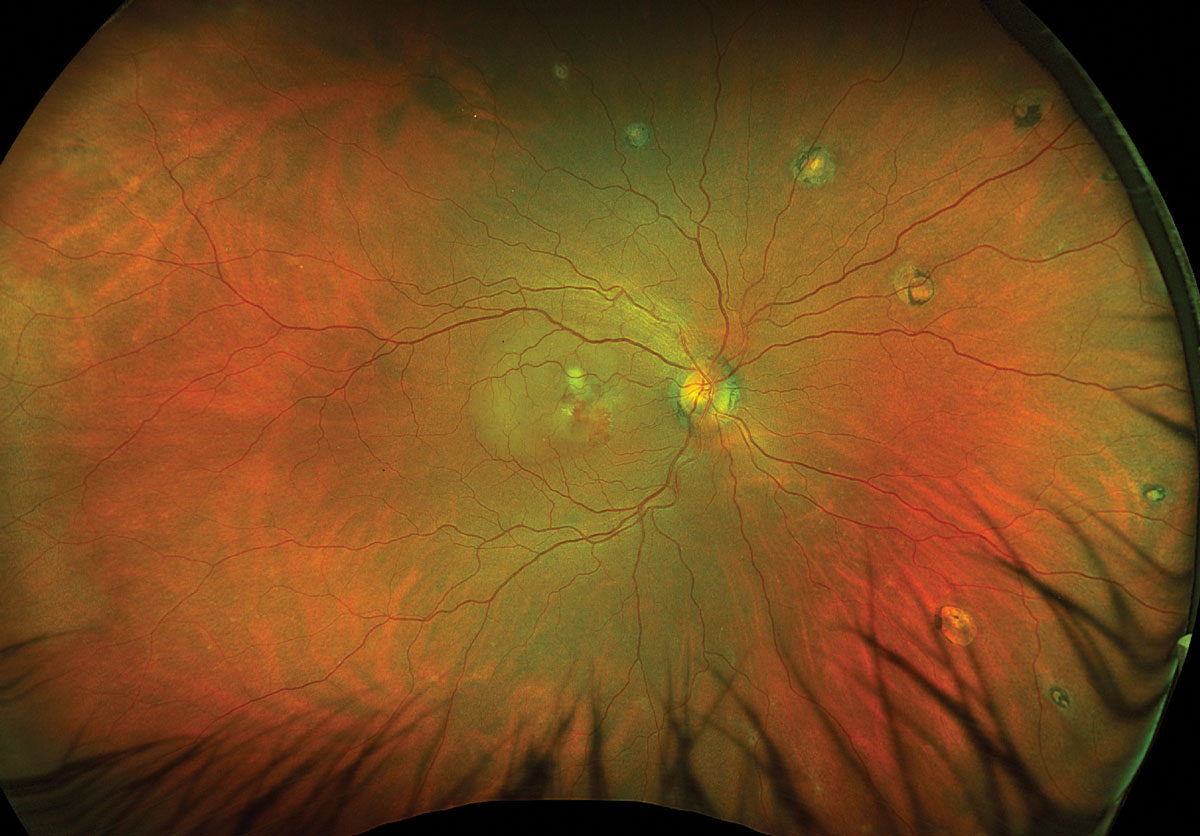 |
Fig. 1. Optos ultrawidefield fundus photo of the right eye. Click image to enlarge. |
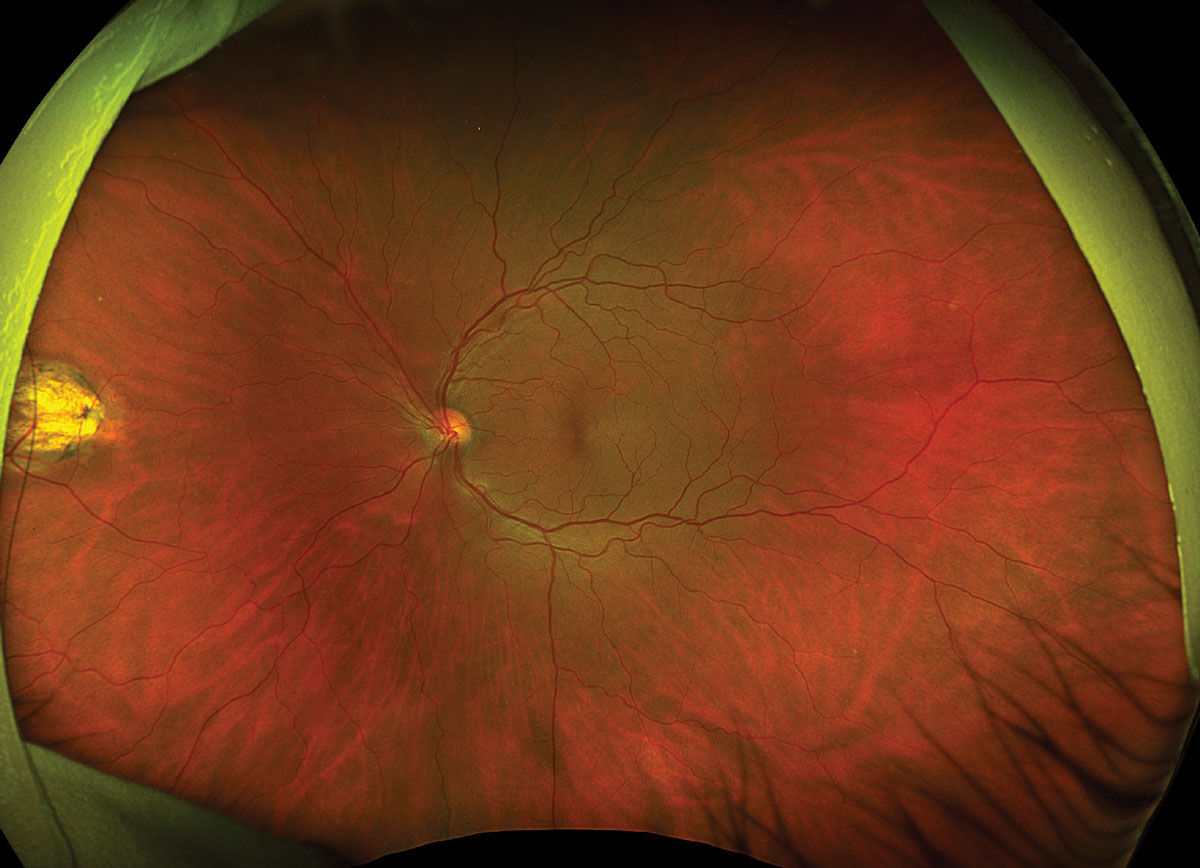 |
Fig. 2. Optos ultrawidefield fundus photo of the left eye. Click image to enlarge. |
Diagnosis
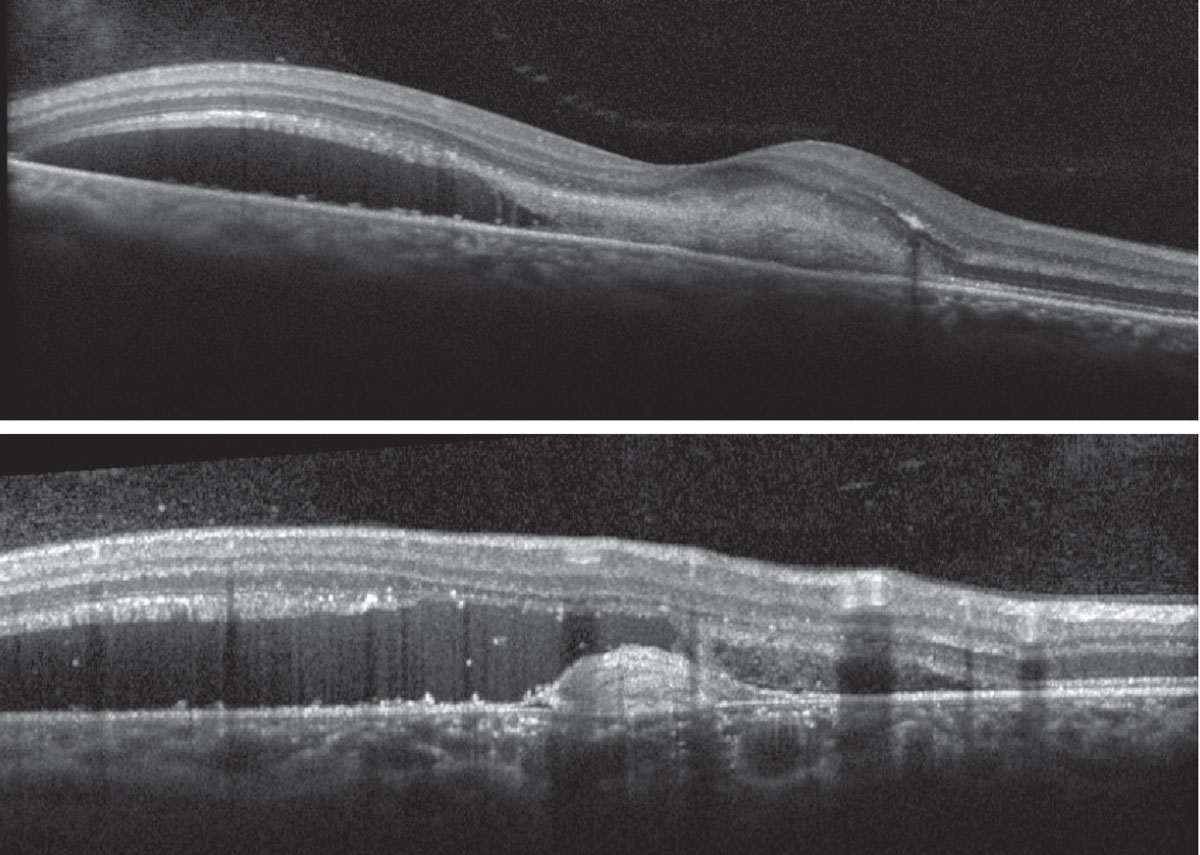
Fundus exam disclosed mild peripapillary atrophy OD, atrophic chorioretinal lesions dispersed throughout the midperipheral and peripheral fundus OU (only partially pictured OS) and subretinal fibrovascular membrane with accompanying subretinal fluid and hemorrhage OD (Figures 1 and 2). OCT confirmed subretinal fluid and hyperreflective material as well as a subretinal pigment epithelial hyperreflective lesion OD; macular OCT was normal OS (Figures 3 and 4).
The constellation of findings in a patient from a known endemic region of histoplasmosis suggested a diagnosis of presumed ocular histoplasmosis syndrome (POHS) with active MNV.
POHS is due to systemic infection by inhalation of Histoplasma capsulatum fungal spores present in soil contaminated by bat and bird droppings.1 Primary infection is pulmonary, and this condition is thought to occur via hematogenous dissemination to the richly vascularized choroid.2 It is termed “presumed” because there are no specific laboratory tests to confirm the ocular manifestations are secondary to histoplasma infection; however, there appears to be a positive association between POHS and histoplasmin skin testing positivity.3,4
In endemic regions, as much as 70% of the population with known exposure to the fungus had positive skin tests, and 100% of those with clinical findings consistent with POHS had positive skin tests. Conversely, only 4.4% of patients from the general population in non-endemic areas with positive histoplasmin skin testing had ocular findings consistent with a diagnosis of POHS.4
Histoplasmosis is the most common endemic mycosis in the world, with case reports spanning five continents (North America, South America, Africa, Europe and Asia).5 In North America, it is considered to be hyperendemic in the Midwestern US (Ohio and Mississippi River Valley) but is also highly endemic to South and Central America with 30% to 40% and 37% to 56% histoplasmin skin testing positivity, respectively.5,6 As a result, it is a leading cause of irreversible vision loss in these regions.1,7 Histoplasmosis is also one of the most frequent opportunistic infections in those infected with human immunodeficiency virus (HIV).5
It is worth noting that 10% of POHS cases have been reported outside of known endemic areas, suggesting that clinicians must maintain an index of suspicion despite absence of supportive travel history; this may be related to climate change and subsequent altered migratory patterns of bats and birds.5,8 Additionally, an association with human leukocyte antigen subtypes DRw2 and B7 has been reported, which supports the hypothesis that the clinical manifestations may actually be an autoimmune reaction secondary to the infectious organism, though further pathophysiological studies are needed.4
The prevalence of POHS in the US is as high as 5.3%, but this is likely an underestimate due to its relatively asymptomatic nature until the development of MNV.2 It typically occurs in the fourth to fifth decades of life with no gender predilection, which may portend socioeconomic challenges given it predominantly affects patients of working age.2,4
The differential diagnosis is very broad and varies based on the presence of MNV; it includes toxoplasma retinochoroiditis, sarcoidosis, punctate inner choroiditis, multifocal choroiditis, multifocal choroiditis with panuveitis, myopic degeneration, age-related macular degeneration, angioid streaks and acute posterior multifocal placoid pigment epitheliopathy.2,4,7,9
As the diagnosis is essentially clinical, a detailed case history and careful ophthalmic exam is critical. POHS is frequently bilateral and the classic findings are a triad of “punched out” chorioretinal lesions affecting the macula or midperipheral/peripheral fundus (also known as “histo spots”), peripapillary atrophy and MNV or subsequent disciform scarring all in the absence of vitritis.4,7,9 Manifestation of two findings of the triad are typically sufficient to make the diagnosis.9 Due to its asymptomatic nature in the early uveitic phase, acute chorioretinal lesions are rarely seen but would appear as discrete white foci that later atrophy and acquire pigment.2,10
Acute MNV formation often produces serosanguinous retinal detachment and subsequent subretinal fibrosis.2,10 While less frequent and described in only about 5% of patients, a fourth clinical sign of pigmented curvilinear streaks in the equatorial fundus was described in 1981 and termed Schlaegel lines.11
Prior to the advent of OCT, fluorescein angiography was the only imaging modality to confirm the presence and activity of MNV.12 However, spectral domain and swept source OCT now allow for more sensitive and early detection of MNV activity, and OCT-angiography has proven a safe, effective and less-invasive alternative modality to fluorescein angiography for assessing MNV activity.2,12
 |
|
Fig. 3. Heidelberg Spectralis OCT of the right macula (foveal and extrafoveal scan). Click image to enlarge. |
Treatment
The Infectious Diseases Society of America concluded that systemic antifungal treatment is not indicated for mild-moderate acute pulmonary histoplasmosis.13 However, systemic antifungal therapy with itraconazole may be indicated for patients with severe pneumonia, chronic pulmonary histoplasmosis, disseminated disease or compromised immune system.13 Ophthalmologically, treatment is only indicated in the presence of active MNV.2,4,7
Historically, laser photocoagulation, photodynamic therapy, submacular surgery and macular translocation were all trialed with variable success, but intravitreal anti-VEGF has become the standard first-line therapy since the initial successful case report in 2007.2,7,9,14 Over 80% of patients treated with intravitreal anti-VEGF show stability or improvement, with an average improvement of about three lines of Snellen best-corrected visual acuity (BCVA); average final BCVA of 20/40-20/60 is achieved with a mean of seven injections over a two-year period.2,3,7
Eyes with suboptimal response to intravitreal anti-VEGF monotherapy may be candidates for combination therapy with PDT.2,4,7,10 A small retrospective series showed a possible role for intravitreal steroid therapy though carries a risk of cataract development and ocular hypertension in a young, working age population.2,4,9 Patients with MNV in one eye have a 12% and 22% chance of developing symptoms in the fellow eye at years five and 10, respectively.10
Our patient is still receiving serial intravitreal bevacizumab injections with trending improvement in the subretinal fluid and hemorrhage and mild eccentric subretinal fibrosis. She has received four injections so far with a BCVA of 20/40 at last follow-up. She will continue with this treatment until resolution and will then require monitoring for reactivation of the neovascular lesion, as well as involvement in the fellow eye.
This case was a reminder that histoplasmosis is truly a diffusely endemic infection that can present in patients outside of the Midwestern US.
Retina Quiz Answers
1: b, 2: d, 3: d, 4: a, 5: d
Special thanks to Alberta Pengo for contributing to this case.
Dr. Aboumourad currently practices at Bascom Palmer Eye Institute in Miami. He has no financial disclosures.
1. Xu TT, Reynolds MM, Hodge DO, Smith WM. Epidemiology and clinical characteristics of presumed ocular histoplasmosis in Olmsted County, Minnesota. Ocul Immunol Inflamm. 2022;30(5):1039-43. 2. Ryan SJ, Davis JL, Flynn HW, et al. Retina, Fifth ed. London; New York: Saunders/Elsevier, 2013. 3. Benedict K, Shantha JG, Yeh S, et al. Presumed ocular histoplasmosis syndrome in a commercially insured population, United States. PLoS One. 2020;15(3):e0230305. 4. Prasad AG, Van Gelder RN. Presumed ocular histoplasmosis syndrome. Curr Opin Ophthalmol. 2005;16(6):364-8. 5. Messina FA, Giusiano G, Santiso G. Endemic mycoses and COVID-19: a review. Current Fungal Infection Reports. 2022;16(3):98-106. 6. Ashraf N, Kubat RC, Poplin V, et al. Re-drawing the maps for endemic mycoses. Mycopathologia. 2020;185(5):843-65. 7. Thuruthumaly C, Yee DC, Rao PK. Presumed ocular histoplasmosis. Curr Opin Ophthalmol. 2014;25(6):508-12. 8. McKinsey DS, Pappas PG. Histoplasmosis: Time to redraw the map and up our game. Clin Infect Dis. 2020;70(6):1011-3. 9. Diaz RI, Sigler EJ, Rafieetary MR, Calzada JI. Ocular histoplasmosis syndrome. Surv Ophthalmol. 2015;60(4):279-95. 10. Agarwal A, Gass JDM. Gass’ Atlas of Macular Diseases, Fifth ed. London: Elsevier, 2011. 11. Fountain JA, Schlaegel TF, Jr. Linear streaks of the equator in the presumed ocular histoplasmosis syndrome. Arch Ophthalmol. 1981;99(2):246-8. 12. Liu TYA, Zhang AY, Wenick A. Evolution of choroidal neovascularization due to presumed ocular histoplasmosis syndrome on multimodal imaging including optical coherence tomography angiography. Case Rep Ophthalmol. Med 2018;2018:4098419. 13. Wheat LJ, Freifeld AG, Kleiman MB, et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clinical Infectious Diseases. 2007;45(7):807-25. 14. Adán A, Navarro M, Casaroli-Marano RP, et al. Intravitreal bevacizumab as initial treatment for choroidal neovascularization associated with presumed ocular histoplasmosis syndrome. Graefes Arch Clin Exp Ophthalmol. 2007;245(12):1873-5. |

