This is the first “On the Horizon” article, an occasional series that will profile unique technologies and innovations in the pipeline.
A Silicon Valley medical device start-up company has recently presented information on a novel, tabletop X-ray delivery system under development for the treatment of choroidal neovascularization (CNV) secondary to age-related macular degeneration (AMD). The Oraya Therapeutics IRay system delivers radiotherapy to the fovea using a low-energy X-ray source in a non-invasive, office-based procedure.
Why X-Ray for AMD?
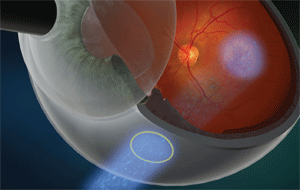
The IRay radiation beam path goes through the sclera and onto the macula.
Age-related macular degeneration, in its advanced form, is a leading cause of blindness in Europeans and those of European descent who are older than 55 years, although it affects younger persons as well. The CNV, or “wet,” form is estimated to affect 1% of U.S. adults, or about 1.2 million people.1
Multiple therapies are advocated for the treatment of wet AMD. The primary treatments currently used are intraocular injections of the vascular endothelial growth factor (VEGF) inhibitors Lucentis (ranibizumab, Genentech) and Avastin (bevacizumab, Genentech). The initial and maintenance treatment protocols for VEGF inhibitor treatment are varied—studies show improved visual acuity with either continuous monthly injections or with three monthly injections at the start of treatment followed by monthly monitoring and retreatment when active disease is detected.2
The treatment protocols, while effective, have a significant impact on the patient, practice and medical payer due to the frequency and cost of treatment and monitoring. Researchers continue to investigate alternative therapies to find better efficacy and durability, including combination therapies.
Some of these alternative therapies involve radiation delivered to the fovea, in combination with VEGF-inhibitor injections.
Radiotherapy to the eye has been well established in the literature and employed extensively in clinical oncologic practice. Toxicity limits have been described for key ocular structures, and they are generally noted to be up to 50 Gray to the central retina, when given in divided doses. (A Gray, or Gy, is a measurement of the absorbed dose of radiation.) Tolerance of the sclera to ionizing radiation is at least as high.3,4
Successful and side effect-free treatment of pterygia with beta-radiation has been reported in single scleral doses up to 30 Gy, and up to 50 Gy in four weekly fractions.5 Studies of pterygia are also suggestive of the potential efficacy of radiation to treat AMD, as they describe the transition from a vascular lesion to a relatively avascular one over the course of the investigation, typically up to several years.
|
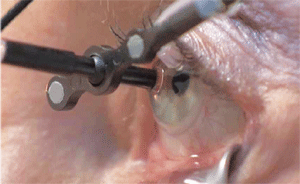
| The IRay’s I-Guide sclera interface stabilizes the eye and also tracks eye movement. |
Oraya’s IRay system is an investigational device that stereotactically delivers low-voltage X-rays to the fovea for the treatment of CNV due to AMD. It is designed to be an easy-to-use, office-based platform for the physician. Its small footprint of 1.5m by 2m allows it to be placed in a modest-sized treatment room. The highly collimated X-ray beam geometry and integrated X-ray shielding provide radiation safety for the clinical staff and obviate the need for extrinsic radiation protection.
The low-energy X-ray tube, similar to what is used in a portable chest X-ray system, produces a collimated, narrow beam designed to affect only the target lesion. It is mounted in a completely self-contained robotic positioning system that ensures precise entry of energy into the eye, avoiding critical structures such as the lens and optic nerve. A treatment-planning model uses globe axial length and fovea location identified through optical coherence tomography to calculate the exact required beam positioning.
The patient’s eye is aligned to the IRay and stabilized using the I-Guide sclera interface. The I-Guide incorporates a sterile, disposable “eye cup” that is coupled to the eye via suction. By incorporating optical fiducials, which are precision reference marks that “communicate” with the positioning system, it holds the eye in the appropriate treatment location. The robotic control system continuously tracks eye movement during treatment, and interrupts the treatment if eye movement or alignment is out of the prescribed limits.
The system can deliver up to 24 Gy to the macula in six minutes using three overlapping 4mm beams, which project through the pars plana via distinct scleral entry positions. The beam path and stereotactic delivery methodology are designed to avoid delivering radiation to sensitive eye structures such as the lens and optic nerve, and maintain scleral radiation levels well below the tolerated limits.
The IRay treatment starts with a planning step that includes axial length measurement and identification of the fovea through OCT. This information is entered into the IRay system, which calculates the beam positioning and customizes it to the patient. The patient’s eye is topically anesthetized, the I-Guide is positioned on the eye, the lower lid is retracted, and then the patient is secured in a chin-rest and head restraint, which gently immobilizes the patient’s head to eliminate unwanted motion. The I-Guide is used to align the eye to the IRay robotics, finalizing the patient preparation for treatment. The total treatment time, including the patient alignment, is no more than 20 minutes.
The patient’s safety is ensured by the eye tracking and gating system, along with safety interlocks that can be tripped by either the patient or operator, stopping radiation delivery. The I-Guide and head restraint are also designed for the patient to rapidly disengage, also stopping the therapy.
The IRay system has been used clinically in more than 100 patients to date. This includes more than 60 in Oraya’s fully-enrolled human pilot study, now in its second year of follow-up, and more than 40 in the first-ever randomized, masked, sham-controlled study of radiotherapy in wet AMD, being conducted in Europe.6 The principal investigators reported positive safety and efficacy data at ophthalmic conferences earlier this year, and have seen no serious treatment-related adverse events thus far.7 The company is planning to start enrolling patients in its U.S. FDA pivotal trial in mid-2010.
Radiotherapy for wet AMD has the potential to positively impact AMD treatment by improving outcomes compared to using anti-VEGF alone, decreasing the treatment burden, and reducing overall treatment costs.
Ongoing clinical trials hope to show these benefits, which if proven should make the management of wet AMD more promising and less burdensome on the patient and the physician.
Dr. Iravani is the founder and chief executive officer of Global EyeVentures, a consulting firm for the ophthalmic industry. Based in San Jose, Calif., in the core of Silicon Valley, Dr. Iravani has a unique opportunity to work with high-tech start-up companies and ophthalmic incubators that hold the promise of delivering new and exciting technology and solutions to eye care. She has no financial interest in any products mentioned.
1. Friedman DS, O’Colmain BJ, Muñoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004 Apr;122(4):564-72.
2. Mitchell P, Korobelnik JF, Lanzetta P, et al. Ranibizumab (Lucentis) in neovascular age-related macular degeneration: evidence from clinical trials. Br J Ophthalmol. 2010 Jan;94(1):2-13.
3. Gordon KB, Char DH, Sagerman RH. Late effects of radiation on the eye and ocular adnexa. Int J Radiat Oncol Biol Phys. 1995 Mar 30;31(5):1123-39.
4. Marcus DM, Sheils W, Johnson MH, et al. External beam irradiation of subfoveal choroidal neovascularization complicating age-related macular degeneration: one-year results of a prospective, double-masked, randomized clinical trial. Arch Ophthalmol. 2001 Feb;119(2):171-80.
5. Pajic B, Greiner RH. Long term results of non-surgical, exclusive strontium-/yttrium-90 beta-irradiation of pterygia. Radiother Oncol. 2005 Jan;74(1):25-9.
6. Oraya website. Press release: Oraya Therapeutics Granted European CE Mark for the IRay Stereotactic Radiotherapy System. Available at www.orayainc.com/press.asp (accessed July 26, 2010).
7. Morales-Canton V, Velez-Montoya R, Moshfeghi DM, et al. Oraya IRay System: Prospective case series of radiation therapy for naïve patients with choroidal neovascularization secondary to age-related macular degeneration as a primary therapeutic approach. Abstract A132. Poster presented at Association for Research in Vision and Ophthalmology, May 2, 2010; Fort Lauderdale, Fla.

