Thyroid eye disease (TED) is the most common orbital disease in North America and is frequently associated with Graves’ disease.1 Although TED often occurs in patients with hyperthyroidism, it is a distinct disease, and treating the underlying systemic thyroid dysfunction often does not resolve the ocular signs and symptoms. At the root of this condition’s pathophysiology is the activation of orbital fibroblasts by autoantibodies, which leads to orbital inflammation early in the disease and subsequent fibrosis.
TED has long been a disease of “watching and waiting,” as traditional treatments are fraught with poor response rates and significant side effects. Early surgical intervention, reserved for severe cases involving vision loss, focuses on controlling inflammation, but patients often still require surgical rehabilitation after reaching the fibrotic phase. Recent new advances in therapeutic options, however, provide promising options to treat the proptosis, inflammation and diplopia that can cause significant patient morbidity in TED.
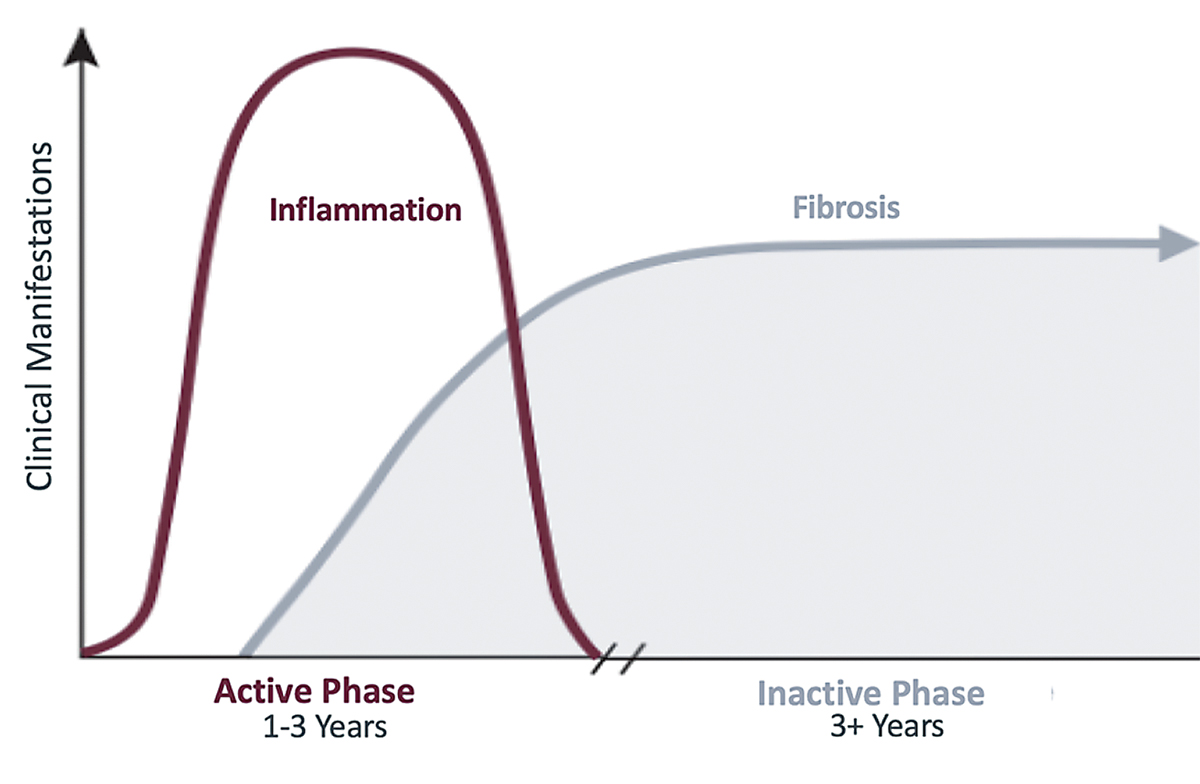 |
| The disease process of TED usually follows the trajectory of Rundle’s curve. Click image to enlarge. |
The Phases of TED
The ophthalmic manifestations of TED begin with an active, inflammatory phase that worsens until reaching a point of maximum severity before leveling off at a static plateau. This disease process typically follows a curve commonly known as Rundle’s curve, which demonstrates the significance of early initiation of therapy in the active phase to diminish overall disease severity.
Unfortunately, patients often have orbital and eyelid changes that persist after the inflammation resolves due to tissue expansion, which occurs in a confined bony orbit, and fibrotic changes, which occur in the orbit and eyelid during the inflammatory phase. Initiating therapy earlier could decrease the potential for long-term damage resulting from this process.
The active phase is typically a self-limited process that lasts an average of one year in nonsmokers and two to three years in smokers. Although physicians view TED as a self-limiting disease, only 2% of patients consider themselves recovered at the end of this phase.2 This disconnect between the physician and patient demonstrates the need for better treatment options that yield clearer results.
Treatment Shortcomings
Current treatment for TED focuses primarily on supportive and palliative care and includes ocular lubrication, prism glasses for diplopia and lifestyle modifications, such as smoking cessation, selenium and vitamin D supplementation and systemic thyroid disease control.
Once a patient is in the stable phase, some undergo surgical intervention, including orbital decompression, strabismus surgery and eyelid reconstruction. Urgent surgery is reserved for severe situations involving compressive optic neuropathy or extensive corneal exposure.
Oftentimes, overlooked or under-acknowledged in treatment is the chronic ocular discomfort, visual impairment and morbidity rate commonly associated with TED. This disease also severely impacts patients emotionally and psychologically—another area that is usually under-treated.
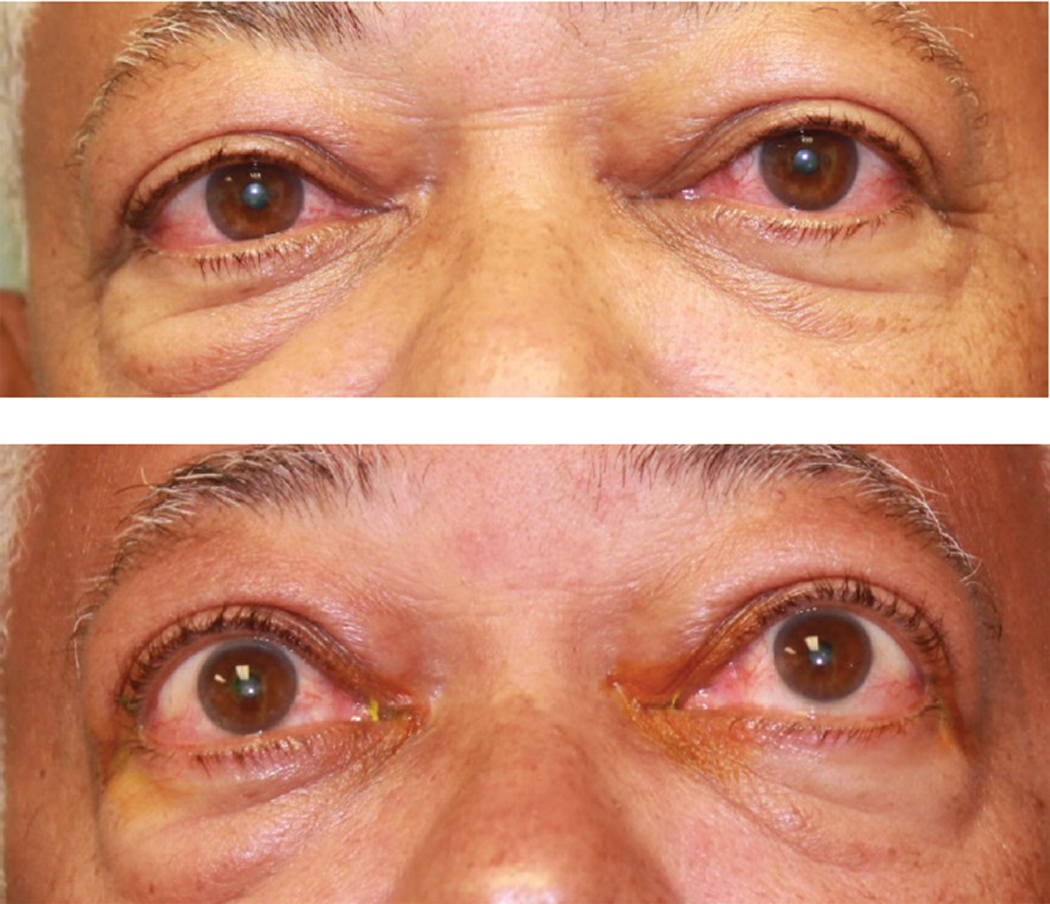 |
Fig. 1. This patient was diagnosed with TED. Click image to enlarge. |
Another Option in the Mix
Historically, there has been little practitioners could do to alter the disease process until research revealed a signaling pathway that involves activation of insulin-like growth factor 1 receptors (IGF-1Rs) in patients with Graves’ disease. This pathway acts synergistically with thyroid-stimulating hormone receptors and enhances their mechanism of action, increasing orbital tissue inflammation.3 Blocking and inhibiting IGF-1Rs can diminish the inflammatory and proliferative process associated with Graves’ ophthalmopathy.
This knowledge led to the recent FDA approval of Tepezza (teprotumumab, Horizon Therapeutics), an antigen-specific therapy designed to block IGF-1Rs and halt the signaling pathway. The medication is “indicated for the treatment of TED.”4 This broad indication offers an advantage, considering the extensive breadth and scope of the disease, and gives providers an option for complex, multifaceted cases.
A Phase III trial found that teprotumumab could significantly reduce both proptosis and diplopia in patients with active, moderate-to-severe TED.5,6 Participants underwent standardized infusions every three weeks for a total of eight treatments, receiving 10mg per kilogram of body weight for the first infusion and 20mg per kilogram for the remaining seven.5,6 At week 24, 83% of patients—compared with 10% of controls—experienced a reduction in proptosis.5,6 Each secondary outcome (overall response, inflammation reduction, proptosis change, diplopia reduction and quality of life score) also faced a more significant improvement with teprotumumab than with placebo.5,6
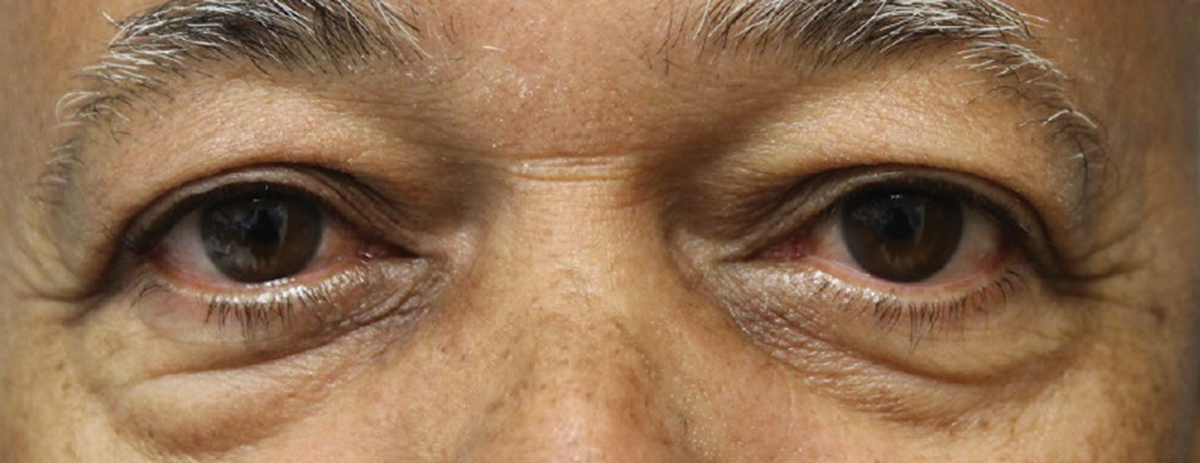 |
| Fig. 2. Visible improvements can be seen in the patient’s eyes after the first Tepezza infusion. Click image to enlarge. |
Additional Considerations
The majority of TED patients are female and in their childbearing years. Given the potential for growth retardation and developmental anomalies, discussing proper contraceptive measures as well as possible pregnancy and fetal development complications with Tepezza is warranted. This medication should not be used in pregnant or lactating women. Contraception is recommended prior to initiating therapy, during treatment and for six months after the last dose of Tepezza.4
While not a contraindication, be sure to warn patients with inflammatory bowel disease (IBD) that there is a risk Tepezza may worsen or cause a flare-up of this condition and counsel them accordingly. A discussion with their gastroenterologist is recommended to assess the severity of their underlying IBD prior to consideration of Tepezza.4
Although there are few adverse events associated with this medication, the most common side effects that providers should be aware of include muscle spasm, alopecia, nausea and fatigue, the majority of which tend to be mild in severity and resolve after treatment.5,6
An adverse effect of special interest documented in the Phase III trial was hearing impairment in five patients (two had hypoacusis, one had deafness, one had autophony and one had mild patulous eustachian tube), which resolved without treatment.5,6 Also during the trial, 10% of patients (two-thirds of who had pre-existing diabetes or impaired glucose tolerance) experienced hyperglycemia, so don’t skimp on monitoring blood sugar levels during the infusion period.4-6 It is also suggested that these patients undergo baseline hemoglobin A1c and fasting blood glucose testing.
As with any infused medication, infusion reactions are rare but possible and affect approximately 4% of Tepezza patients.4 They may occur during or within 1.5 hours after an infusion and range in severity from mild to moderate.4 Signs and symptoms of infusion-related reactions include transient increases in blood pressure, hot flashes, tachycardia, dyspnea, headache and muscular pain. They are usually successfully managed with corticosteroids and antihistamines.4
The Many Faces of TEDIn some cases, TED can be challenging to diagnose. These masquerading signs and symptoms should heighten the clinician’s suspicion that there may be an underlying thyroid basis to a patient’s ocular condition, warranting further history and workup: a. Orbital congestion (not to be mistaken for conjunctivitis) b. Allergic conjunctivitis without any papillary reaction that doesn’t improve with allergy drops c. Unexplained changes in vision that are inconsistent with corneal changes from dryness or other pathologies, which can actually be caused by low-grade chronic compressive optic neuropathy i. Resistance to retropulsion, an unsatisfactory response to a careful motility check and lid lag on downgaze can help with this diagnosis ii. Optic nerve imaging with OCT and visual field testing can be helpful in these cases d. Temporal chemosis with injection overlying the extraocular muscles e. Chronic ocular ache and pain as opposed to the more common sharp pains associated with dry eye and other corneal disorders |
Patient Management
As TED is a multisystem disease, these patients are best managed through integrated care provided by a team of medical professionals from various fields, including endocrinology, oculoplastics, primary care, optometry and infusion clinic staff. Optometrists specifically are well positioned and able to play a key role in the detection, management and ongoing visual care of these patients, as TED is, at its roots, an eye disease.
Monitoring ocular inflammation, proptosis, binocular function and optic nerve integrity is extremely feasible in the optometric setting, offering a clearer clinical picture and placing optometrists at the heart of the equation to serve as the link between other subspecialties.
By intercepting Rundle’s curve early in its surge, we have the chance to prevent severe manifestations that could arise when TED is left untreated. With its FDA approval, Tepezza is in a position to become the standard of care in treating patients with this cosmetically disabling condition. Given the medication’s effectiveness and associated improvements, it is critical that TED patients be offered it as an option.
At a bare minimum, Tepezza has changed how we should look at and think about TED. It shatters the “watch and wait” mentality and challenges practitioners to be on the lookout for the initial signs of this debilitating disease so we can treat it earlier and more effectively than ever before. It’s also encouraging to know there is now something we can offer patients to help modify the course of their disease and improve their quality of life, something these patients—and their doctors—have long been hoping for.
Dr. Lang is an adjunct clinical faculty member at the Illinois College of Optometry and Salus University, the residency coordinator for Associated Eye Care’s optometric residency program in Stillwater, MN, a diplomate of the American Board of Optometry and a fellow of the American Academy of Optometry. He participates in clinical research and FDA trials, writes articles for several publications and lectures at various meetings, with a focus on dry eye and external disease.
Dr. Harris graduated from the Illinois College of Optometry in 2019. She is currently pursuing her residency in ocular disease at Associated Eye Care.
Dr. Wester is an associate clinical professor at the University of Miami Bascom Palmer Eye Institute. She specializes in TED and reconstructive and cosmetic surgery of the eyelids and orbit. She conducts clinical research and trials, has authored many publications, is a consultant for Horizon Therapeutics and speaks at conventions around the world.
The authors wish to thank Aaron Bronner, OD, for his assistance in preparing this manuscript.
1. Liaboe CA, Simmons BA, Clark TJ, et al. EyeRounds. Thyroid eye disease. webeye.ophth.uiowa.edu/eyeforum/patients/thyroid-eye-disease.htm. September 1, 2016. Accessed May 15, 2020. 2. Sabini E, Leo M, Mazzi B, et al. Does Graves’ orbitopathy ever disappear? Answers to an old question. Eur Thyroid J. 2017;6(5):263-70. 3. Tramontano D, Cushing GW, Moses AC, et al. Insulin-like growth factor-I stimulates the growth of rat thyroid cells in culture and synergizes the stimulation of DNA synthesis induced by TSH and Graves’-IgG. Endocrinology. 1986;119(2):940-2. 4. FDA. Tepezza. www.accessdata.fda.gov/drugsatfda_docs/label/2020/761143s000lbl.pdf. Accessed May 15, 2020. 5. Smith TJ, Kahaly GJ, Ezra DG, et al. Teprotumumab for thyroid-associated ophthalmopathy. N Engl J Med. 2017;376(18):1748-61. 6. Douglas RS, Kahaly GJ, Patel A, et al. Teprotumumab for the treatment of active thyroid eye disease. N Engl J Med. 2020;382(4):341-52. |

