 |
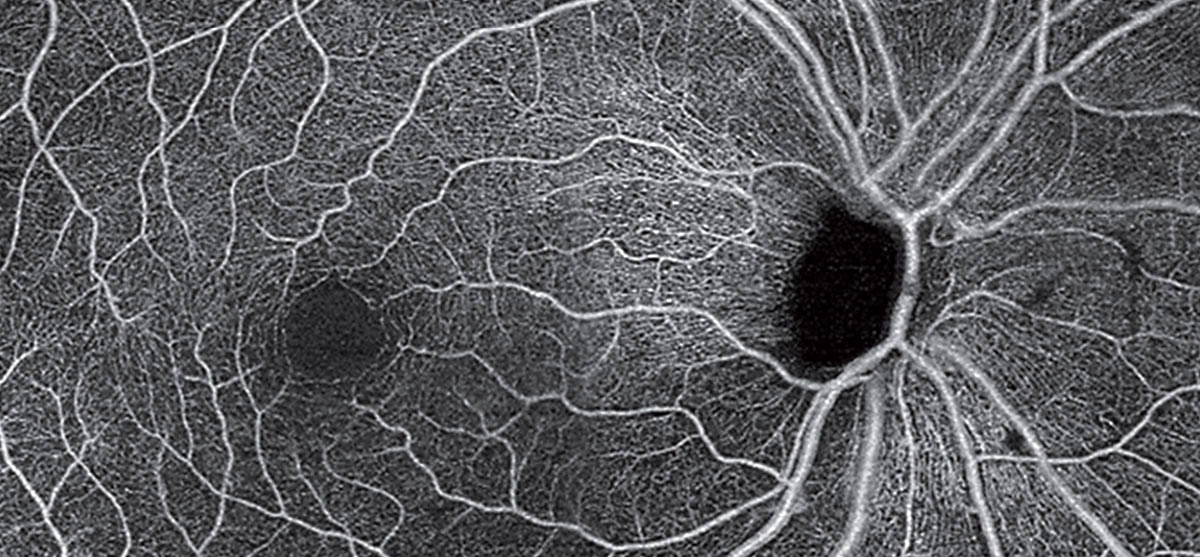
| Parkinson’s may be linked to the eye through cerebrovascular changes affecting different retinal layers. This OCT-A montage of an unrelated patient (with POAG) shows loss of radial peripapillary capillaries and the superficial capillary plexus. Photo: Carolyn Majcher, OD. Click images to enlarge. |
The second-most common neurodegenerative condition, Parkinson’s disease (PD) has increasingly been characterized not only by movement alterations but with various non-motor symptoms as well, including dementia, depression and autonomic dysfunction. Many different visual dysfunctions have also been described in Parkinson’s patients, prompting one new study to clarify the relationship between PD and the retina in hopes that the eye might serve as a way to diagnose the disease early and detect disease progression. A growing body of literature already supports the association but there are many nuances yet to uncover.
The macular superficial capillary plexus (SCP) density, measured with OCT angiography (OCT-A) and ganglion cell complex (GCC) thickness, measured with OCT, were correlated in 38 PD patients and 36 controls. Correlation between SCP density and Parkinson’s clinical parameters was also tested.
When compared with the control group, the Parkinson’s patients displayed reduced SCP density in all macular region areas, including the para- and perifoveal temporal, superior, nasal and inferior areas. The GCC thickness on average was also significantly reduced in these patients. What’s more, there was a positive correlation between average GCC thickness and SCP densities for most of the macular area regions in Parkinson’s patients, suggesting retinal microvascular damage that may be associated with retinal structural degeneration, corresponding with pathological brain damage. Correlation was additionally found between SCP densities and Hoehn and various measures of disability associated with Parkinson’s disease.
The researchers explain that the cerebral and retinal microvasculature both share some characteristics. Both blood supplies come from the internal carotid artery, potentially making cerebral angiopathy in PD extend into the retina. Furthering the connection between the two, recent research has uncovered that cerebrovascular changes are vital in causing Parkinson’s and are also a contributing factor in progression of the disease. As this relates to the retina, a previous study has found retinal vessel density is significantly reduced in PD patients’ macular regions.
Parkinson’s, characterized by selective dopaminergic neuronal loss, may be related to the GCC thinning. Dopaminergic neurons are found in the inner retinal layer as retinal ganglion cells and amacrine cells. The GCC layers of retinal nerve fiber layer, ganglion cell layer and inner plexiform layers anatomically line up with the dendrites, cell bodies and axons of ganglion cells, respectively. The thinner GCC found in this study of PD patients mapped onto dopaminergic neuronal loss of the substantia nigra, meaning that ganglion cells, which are dopaminergic, may be affected by dopaminergic neurodegenerative lesions caused by PD.
Due to these findings, the study authors propose that “although we cannot verify if microvasculature damage could cause retinal neurodegeneration in PD patients, our results suggest that retinal microvascular damage is associated with dopaminergic degeneration in the retina in PD patients.”
They add that this may be beneficial for real-world clinical usage, as “OCT-A and OCT may be potential adjuncts to detect microvascular changes and neurodegeneration in the early stages of PD in the future.”
Zhang L, Zhuang C, Wang Y, et al. Clinical observation of macular superficial capillary plexus and ganglion cell complex in patients with Parkinson’s disease. Ophthalmic Res. August 10, 2023. [Epub ahead of print]. |

