 A 57-year-old white male presented to the office in July 2008 as a new patient after he recently moved to the area. His last eye examination was five to six years earlier. His ocular history is significant for bilateral LASIK in 1998 and one bilateral enhancement procedure in 1999. He had no known history of glaucoma.
A 57-year-old white male presented to the office in July 2008 as a new patient after he recently moved to the area. His last eye examination was five to six years earlier. His ocular history is significant for bilateral LASIK in 1998 and one bilateral enhancement procedure in 1999. He had no known history of glaucoma.
Current medications include lisinopril and Allegra (fexofenadine hydrochloride, Aventis Pharmaceuticals). He reported no known allergies to medications.
Diagnostic Data
Uncorrected visual acuity was 20/40 O.D. and 20/30+ O.S. Best-corrected visual acuity was 20/20-1 O.D. and 20/20 O.S. through a refraction of +1.00D1.25D x 065 O.D. and plano0.75D x 110 O.S. Pupils were equally round and reactive to light and accommodation, with no afferent pupillary defect. Confrontation fields were full in all positions of gaze.
A slit lamp examination of his anterior segments was remarkable for visible corneal flap edges O.U. The edges were smooth, without irregularity, and there was no evidence of epithelial ingrowth in either eye. The central flaps were clear, with no striae, and both right and left flap/bed interfaces were pristine.
The anterior chamber angle, as estimated by Von Hericks method, was grade 4 open O.U. Intraocular pressure (IOP) was 18mm Hg O.D. and 16mm Hg O.S. at 4:00 p.m.
Through dilated pupils, his crystalline lenses were clear O.U. Scattered vitreous floaters were present in both eyes. His cup-to-disc ratio was 0.70 x 0.75 O.D. and 0.65 x 0.70 O.S. The optic nerves were of normal size. The inferior and superior temporal neuroretinal rims O.U. were somewhat thinned with sloping borders, making an assessment of optic nerve health difficult. His retinal vasculature was normal O.U.
Both maculae were characterized by mild retinal pigment epithelium (RPE) mottling, greater O.D. than O.S. His peripheral retinal examination O.U. was remarkable for 360 microcystoid degeneration and scattered areas of paving stone degeneration. No holes or tears were present.
Stereo images of our 57-year-old patients optic nerve O.D. What do you notice?
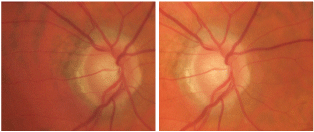
Given the suspicious appearance of the patients optic nerves, we asked him to return for a baseline glaucoma evaluation.
The patient returned four weeks later. At this visit, his IOP was 17mm Hg O.D. and 18mm Hg O.S. at 8:30 a.m. Central corneal thickness (CCT) measured 492m O.D. and 506m O.S. White-on-white visual fields were unremarkable O.U. Gonioscopy demonstrated grade 4 open angles, with mild trabecular pigmentation O.U. Baseline Heidelberg Retina Tomograph-3 (HRT-3) images were obtained O.U., and Moorfields Regression Analysis showed aberrations in both optic nerves.
Discussion
It is well documented that corneal thinning following ablative procedures has an effect on post-surgical IOP readings, particularly on measurements obtained with a Goldmann applanation tonometer (GAT). Because of this effect, a patients postoperative IOP typically will be lower than before surgery.1
As a result, various technologies have been developed to give us a better picture of true IOP in post-LASIK patients, such as the Pascal Dynamic Contour Tonometer [(DCT) Zeimer Ophthalmology] and the Ocular Response Analyzer [(ORA) Reichert Ophthalmic Instruments]. Even older technologies have been re-evaluated to see if they can provide a more accurate measurement of IOP.2
We are now well aware that CCT is a risk factor for glaucoma development and progression. However, the specific role of CCT may vary depending on baseline IOP, race, ethnicity and a family history of glaucoma.
Several studies have examined the role of CCT in post-refractive patients in the context of glaucoma. But, how do you clinically proceed with a patient, as in this particular case, who not only has the appearance of glaucoma, but also has undergone a corneal ablative procedure?
There are two essential questions that need to be answered in this case. First, and most pressing: Does this patient have glaucoma? Then, if the answer to this question is yes (and I believe that our patient is manifesting the early signs of glaucoma), we consider the second question: What is the best way to manage this patient?
Clearly, this is where some of the newer technologies may assist us in both short-term and long-term patient management decisions. The DCT has been studied for years and has been shown to document an IOP measurement that is less dependent on CCT. The ORA has shown similar findings.
However, no discussion of glaucoma and post-LASIK corneas can take place without at least touching on the topic of corneal hysteresis (CH). CH is an indicator of the viscoelastic properties of a cornea. Essentially, this measurement gives us a sense about the flexibility of the cornea.
With a rapid air pulse, the ORA calculates CH through an analysis of the cornea as it bows inward upon exertion of the pulse as well as when the cornea rebounds toward its normal configuration.
In the context of refractive surgery, research suggests that CH gives a clearer picture of what is happening in the cornea postoperatively and may predict potential ectatic problems.3,4
Regarding glaucoma, similar research suggests that CH needs to be evaluated when considering IOP measurements.3,4 Because our patient has not only undergone LASIK, but also is a glaucoma suspect, both an accurate measurement and a thorough understanding of CH is even more imperative.
Using GAT, DCT and ORA, a recent study specifically looked at the effects of CCT and CH on IOP measurement in glaucoma patients.3 This study showed that CH was highly correlated with IOP when measured by ORA, but not when measured by GAT or DCT.
In another study, an assessment of CH was examined in a population of normotensive glaucoma patients, open-angle glaucoma patients and ocular hypertensives.4 This study showed that CH was highest in ocular hypertensives and lowest in normotensives. However, the clinical utility of this data has yet to be determined. Though the relationships between CH, CCT and IOP measurements intuitively make sense, the way that we use the information is subject to individual interpretation.
We must not forget that glaucoma is an optic neuropathy, and as such, a disease of the optic nervenot the cornea. It may sound silly to say, but we cannot get caught up in an over-analysis of the cornea and an under-analysis of the optic nerve.
Would it be helpful to know this patients preoperative CCT and refractive error? In other words, would it be beneficial to know how much corneal tissue was removed so that we can get a sense of what has changed with his IOP from 1998 to the present?
While those pieces of information would be nice to have, I do not think that they are critical to his current management. If you had that information, would you adjust the IOP according to a nomogram? An argument can be made both ways, but it does not change the fact that we have a patient with suspected optic nerve damage.
Whether you choose to medically intervene or passively observe the patient, you must continue to monitor the patients IOP, visual fields and optic nerve characteristics. These three fundamental measurements remain at the core of any glaucoma management strategy and should be continually analyzed for any repeatable change over time. Additional information does not necessarily make your decision any easier.
As for this patient, I do believe that his optic nerves are compromised. We started him on a course of one drop Travatan Z (travoprost, Alcon) h.s. O.U. He is scheduled for follow-up in two weeks.
1. Emara B, Probst LE, Tingey DP, et al. Correlation of intraocular pressure and central corneal thickness in normal myopic eyes and after laser in situ keratomileusis. J Cataract Refract Surg 1998 Oct;24(10):1320-5.
2. Duch S, Serra A, Castanera J, et al. Tonometry after laser in situ keratomileusis treatment. J Glaucoma 2001 Aug;10 (4):261-5.
3. Annette H, Kristina L, Bernd S, et al. Effect of central corneal thickness and corneal hysteresis on tonometry as measured by dynamic contour tonometry, Ocular Response Analyzer, and Goldmann tonometry in glaucomatous eyes. J Glaucoma 2008 Aug;17(5):361-5.
4. Shah S, Laiquzzaman M, Mantry S, Cunliffe I. Ocular Response Analyser to assess hysteresis and corneal resistance factor in low-tension, open-angle glaucoma and ocular hypertension. Clin Experiment Ophthalmol 2008 Aug;36(6): 508-13.

