You probably already know about the treatment burden posed by anti-VEGF therapy for AMD and other retinal diseases—namely, that the remarkable visual acuity gains come at the expense of a lifelong injection regimen. Less well known: Long-term anti-VEGF use may also pose structural risks to the retina—although it is unclear whether these arise from the therapy or the devastating effects of AMD itself.
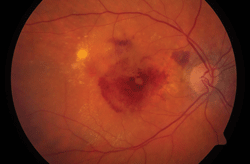
A new study questions whether anti-VEGF treatment does more harm than good.
A study from the Scripps Research Institute, published in this month’s Journal of Clinical Investigation, found mice with their VEGF-producing genes removed experienced deterioration of the choriocapillaris (the major supplier of nourishment to the retinal pigment epithelium), death of cone photoreceptors and corresponding visual loss. This led researchers to speculate that drastic VEGF reduction may do more harm than good. The Scripps team plans follow-up research on human AMD patients and to explore other potential targets for suppressing angiogenesis.
Because VEGF plays a role in choroidal vascular development, it is widely believed to be a contributor to adult retinal health. Research suggests VEGF signaling may help maintain the choriocapillaris.
That gives some retina specialists pause. “It may be possible to induce too much VEGF suppression,” says Pravin Dugel, M.D., a Phoenix-based retinal specialist. “Some neovascularization, some profusion may actually be beneficial, or else we risk trading in one problem for another.” He notes that in the CATT year-two trial results, patients with the driest, thinnest retinas also showed higher incidence of geographic atrophy. Other studies have linked geographic atrophy and photoreceptor cell death with long-term AMD.
Combination therapy may help to ease treatment burden problems as well as maintain VEGF equilibrium, according to Dr. Dugel, who is involved in investigating Fovista (Ophthotech), an anti-PDGF (platelet-derived growth factor) agent, administered simultaneously with Lucentis (ranibizumab, Genentech) anti-VEGF therapy.
At this month’s American Academy of Ophthalmology meeting, Dr. Dugel presented Phase II clinical data that involved 449 subjects. Patients who received Fovista 1.5mg combined with Lucentis 0.5mg experienced a mean increase of +10.6 letters of vision at six months—a 62% improvement over Lucentis monotherapy.
Anti-PDGF and anti-VEGF work synergistically, Dr. Dugel says, with the former stripping a protective layer of pericytes from neovascular endothelial cells to allow the latter to work more effectively at fighting proliferation.
Despite the likely arrival of Fovista and other agents that might outperform anti-VEGF alone, no hard evidence exists to confirm that long-term anti-VEGF therapy poses health risks in humans.
“It is difficult if not impossible to distinguish between atrophic damage that occurs in the natural course of the disease and theoretical atrophic damage that might occur from anti-VEGF therapy, and I’m not aware of any such evidence in human studies,” explains Robert Bhisitkul, M.D., professor of clinical ophthalmology at the University of California, San Francisco, who has studied the subject.
He makes a distinction between “geographic atrophy” that occurs in dry AMD, and non-specific damage to the retina and RPE that occurs in wet AMD.
“Geographic atrophy in dry AMD is like termites, whereas atrophic damage in wet AMD is like water or fire damage—both can result in damage to your house, but they are different processes,” Dr. Bhisitkul says. In neovascular AMD, “you’ve got fluid and blood poured onto the retina, causing mechanical damage; you’ve got blood vessels burrowing into the RPE, causing destruction.”
Kurihara T, Westenskow PD, Bravo S, et al. Targeted deletion of VEGFA in adult mice induces vision loss. J Clin Invest. 2012 Nov 1;122(11):4213-7.

